Figure 1. Overview of genetic pathways in nonalcoholic fatty liver disease (NAFLD).
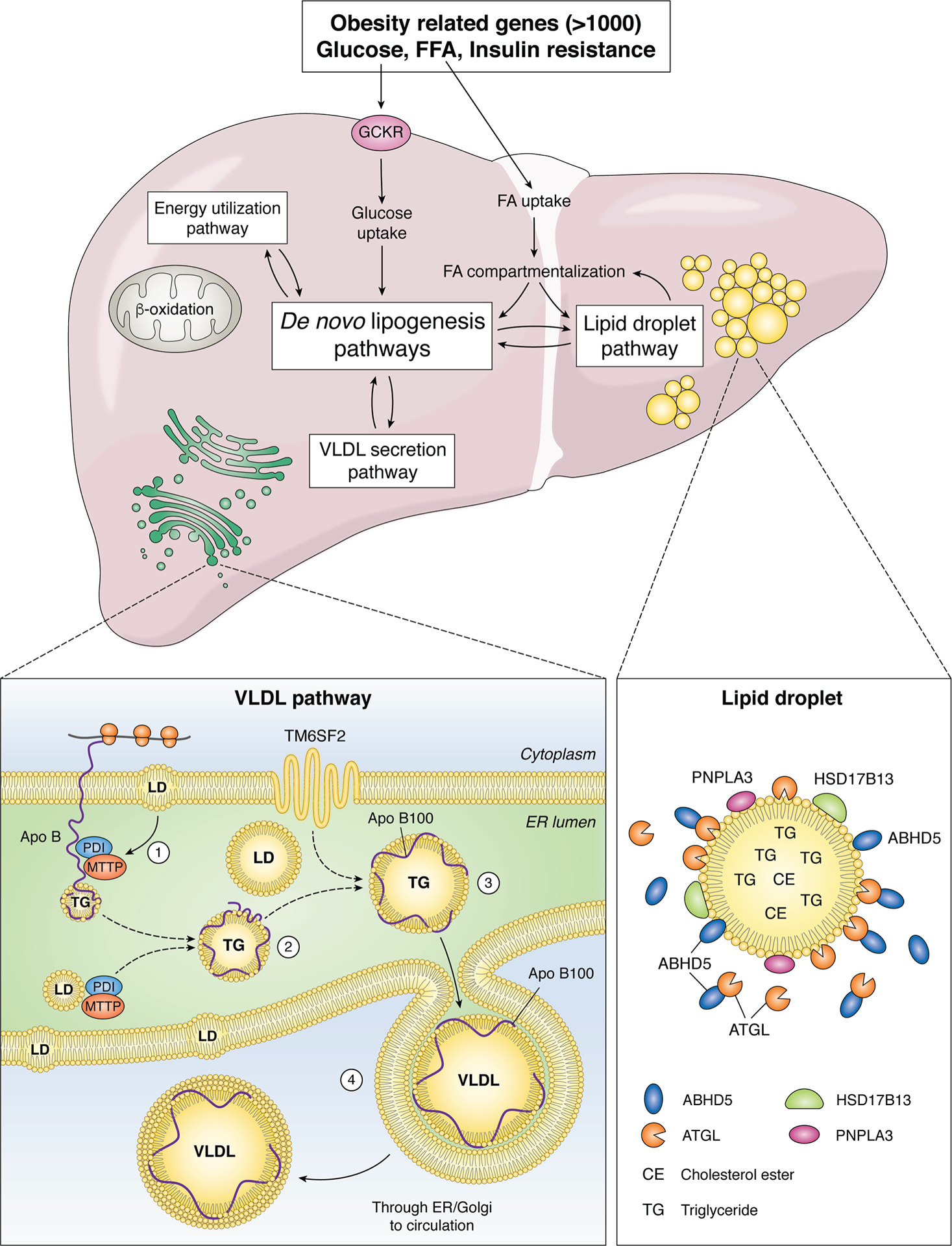
The major pathways involved in lipid trafficking, compartmentalization and utilization are represented within boxes, each of which undergo regulation through a combination of host genetic factors and environmental interactions (14). Obesity and insulin resistance are each complex genetic traits, with variants in more than 1000 genes linked to altered susceptibility (106, 107). Variations in energy metabolism are linked to variants in mitochondrial genes including uncoupling protein 1, 2 (UCP1, UCP2) as well as superoxide dismutase2 (SOD2), whose candidate genes are expressed in extrahepatic tissues (adipose, muscle) as well as the liver. Fatty acid uptake and metabolic channeling results in partitioning between de novo lipogenesis/energy utilization/lipid droplet pathways. Hepatic glucose uptake and utilization is also regulated by variants in glucokinase regulator (GCKR) (61), which in turn regulate substrate flow for de novo lipogenesis. In addition, de novo lipogenesis pathways also interact metabolically with the very low density lipoprotein (VLDL) secretion pathway. The lipid droplet (LD) pathway includes many of the candidate genes implicated in NAFLD development and progression, including patatin-like phospholipase domain-containing protein 3 (PNPLA3), abhydrolase containing domain 5 (ABDH5), adipose triglyceride lipase (ATGL), hydroxysteroid 17-β-dehydrogenase B13 (HSD17B13). Those proteins are associated with LDs which contain a core of neutral lipids (triglyceride, TG and cholesterol ester, CE). The VLDL pathway includes gatekeeper genes (microsomal triglyceride transfer protein, MTTP and apolipoprotein B, APOB, variants of which impair VLDL assembly within the endoplasmic reticulum (ER). MTTP is an endoluminal ER protein that functions as an obligate heteromeric complex with protein disulfide isomerase (PDI) and together promote lipidation and correct folding of the APOB protein around a core of neutral lipid transferred from membrane associated and intraluminal LD. In addition, variations in another transmembrane ER associated protein, transmembrane 6 superfamily 2 (TM6SF2) are associated with defective VLDL assembly and secretion (74, 75).
