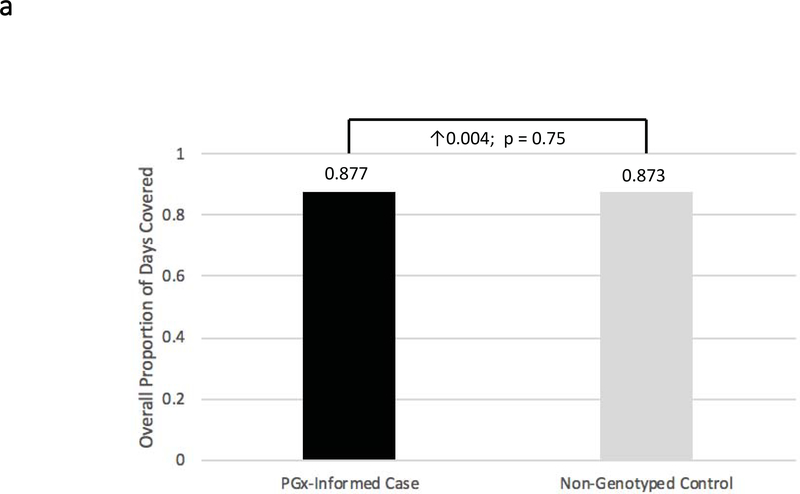
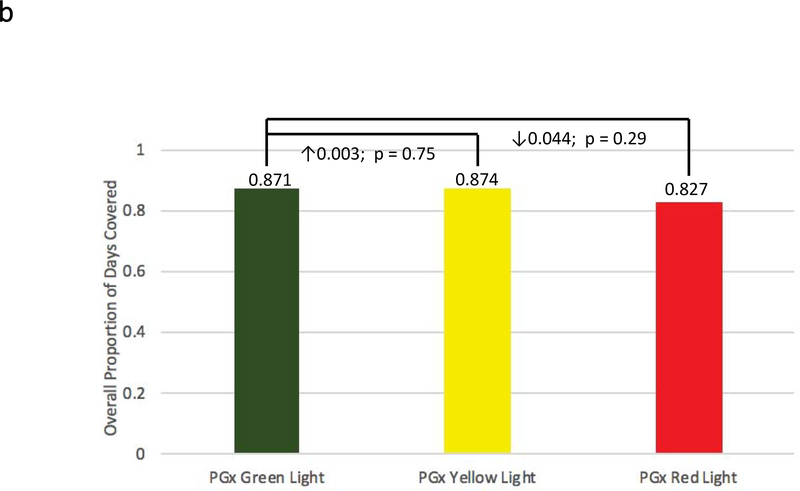
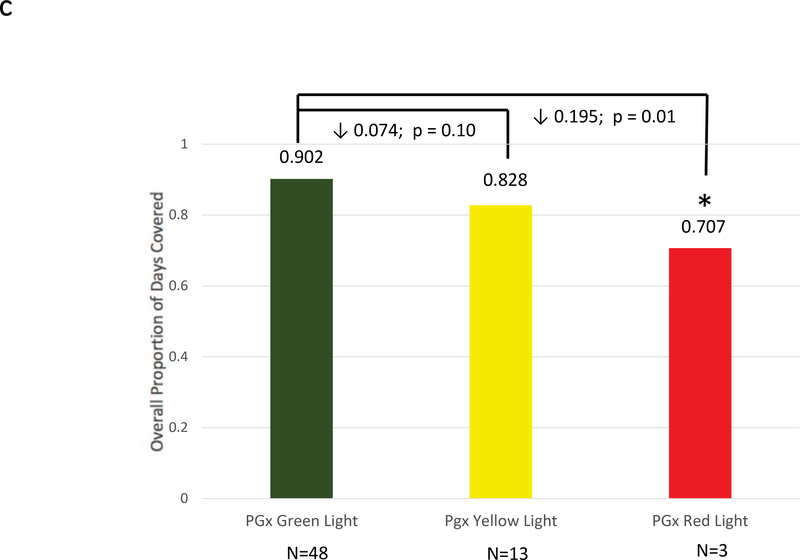
Figure 2: Adherence Comparisons between Genotyped Cases and Non-Genotyped Controls.
Adherence was defined as the mean composite modified proportion of days covered (mPDC) for all evaluable medications. (a) Comparisons were made between pharmacogenomic informed case patients (N = 270) and non-genotyped control patients (N = 266) using all medications reported within GPS for which e-prescriptions were written. (b) Comparisons were made between cases that were prescribed genomically-concordant (green light), cautionary (yellow light), and genomically-incongruent, high-risk (red light) medications, using all medications reported within GPS. (c) Comparisons were made between cases that were prescribed genomically-concordant (green light), cautionary (yellow light), and genomically-incongruent, high-risk (red light) medications, using all medications reported within GPS that have CPIC A or CPIC B designations. GPS = Genomic Prescribing System; CPIC = Clinical Pharmacogenetics Implementation Consortium.