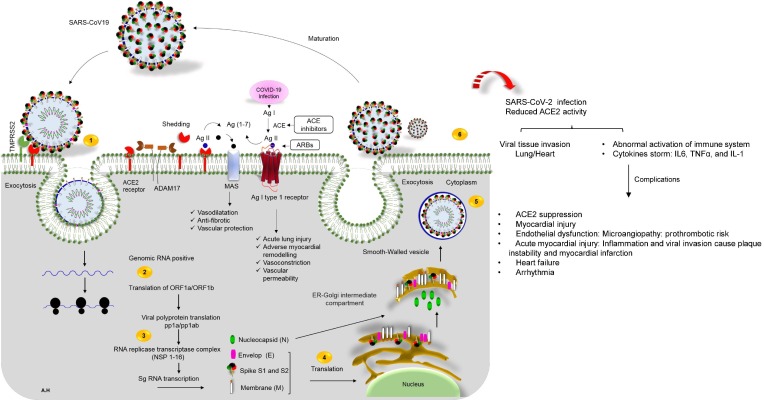
Fig. 2.
The life cycle of SARS-CoV-2 in host cells, and cardiovascular complications of virus infection. 1) ACE2 binds to the viral S protein, which is cleaved into two subunits, S1 and S2, by an extracellular protease. While S1 binds to ACE2, S2 is further cleaved, and activated by serine protease TMPRSS2. Together these actions result in host-viral membrane fusion, and the release of the RNA genome into the host cell cytoplasm, 2) Translation of structural, and non-structural proteins as follows: ORF1a, and ORF1ab proteins are translated to yield pp1a, and pp1ab polyproteins. These are cleaved by proteases that are encoded by ORF1a to produce 16 non-structural proteins. The polyproteins (pp1a, and pp1ab) are cleaved into 16 non-structural effector proteins by 3CLpro, and PLpro, 3) allowing them to form the replication complex together with the RNA polymerase, which synthesizes a full-length negative RNA strand template, 4) This template is used to replicate the complete RNA genome, and generate the individual sub-genomic mRNA needed for the translation of the viral structural, and accessory proteins, 5) The newly synthesized structural, and accessory proteins are then trafficked from the ER through the Golgi apparatus, after which new virions assemble in bourgeoning Golgi vesicles, 6) Finally, the mature SARS-CoV-2 virions are exocytosed, and released from the host cell. Also shown, schematic representation of the RAS-pathway in which ACE/Ang-II, and ACE/Ang 1–7/Mas-axis. Surface ACE2 is further down-regulated, resulting in unopposed angiotensin II accumulation describing the regulatory role of ACE2-Ang 1–7 axis in the cardiovascular homeostasis. Local activation of the RAAS by SARS-CoV-2 spike may mediate lung injury, and responses to viral insults such as CV complications. ACE enzyme inhibitors and angiotensin receptor blockers (ARBs) possibly rise the risk of severe COVID-19 outcomes. On the right of the panel, local complications of virus infection on cardiovascular system is presented.