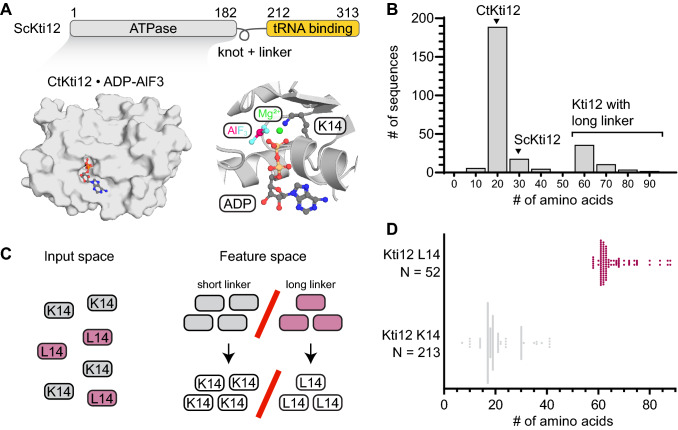
Fig. 1.
Identification of Kti12 proteins with elongated linker and their characterization. a Architecture of Kti12 protein. ATPase domain (grey) and tRNA-tethering domain (yellow) are connected by a flexible linker. Structural overview of Chaetomium thermophilum Kti12 ATPase domain (PDB ID: 6QP0) with a particular emphasis on nucleotide binding pocket and K14 located in a p-loop. b Distribution of linker length across fungal Kti12 proteins. Numbers on Ox indicate middle values for particular baskets, for instance, 18 ± 4. Black triangles indicate groups to which ScKti12 and CtKti12 belong to. c Scheme of supervised a machine-learning experiment that pinpointed coincidence of L14 with elongated linkers. Sequences with linkers of a regular length (grey) always carry lysine at position 14 (K14), whereas Kti12 proteins with elongated linkers (pink) can be distinguished by the presence of leucine in a p-loop (L14). d Violin plots represents distribution of Kti12 protein sequences in a two-dimensional feature space. Linker length is on Ox whereas presence of leucine or lysine qualifies sample to one of the groups on Oy axis. Number of sequences within each group is indicated next the Oy axis