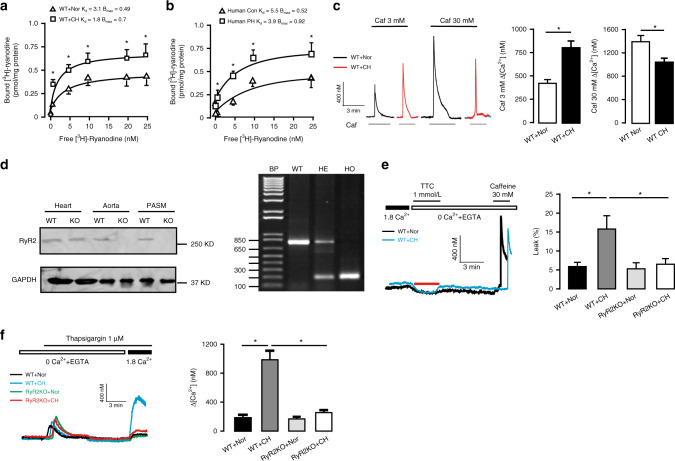
Fig. 1. Deletion of RyR2 gene prevents CH-induced calcium signal over-activation.
a [3H]-ryanodine binding assays are conducted from wild-type (WT) mice pulmonary arteries (PAs) pretreated with normoxia (Nor) and chronic hypoxia (CH) (n = 5 independent studies, 5 mice per study). b Human PAs samples with pulmonary hypertension (PH) and age-matched non-PH human PAs (Con) are used to evaluate RyRs activity by [3H]-ryanodine binding assays (n = 3 independent studies). c Recording of medium (3 mM) and maximal (30 mM) concentration caffeine (Caf)-induced PASMC Ca2+ release. Bar graph summary of medium (3 mM) and maximal (30 mM) concentration Caf-induced Ca2+ release in WT pulmonary arterial smooth muscle cells (PASMC) after exposure to 3 weeks CH (n = 7 independent studies, 95 cells per study). d Representative western blot and PCR of RyR2 expression from RyR2 gene knockout (KO) mice. Tissue are harvested from heart, aorta and PASM (n = 3 independent studies, 4 mice per study). e Representative [Ca2+]i tracing from Nor and CH PASMC loaded by fura-2/AM switch to 0 Ca2+ physiological saline solution (PSS) and 1 mmol/L tetracaine (TTC) to block RyR2. Sarcoplasmic reticulum (SR) Ca2+ content is measured by adding 30 mM caffeine. SR Ca2+ leak ratio is quantified and normalized to SR Ca2+ content in WT and RyR2 KO mice at Nor and CH condition (n = 4 independent studies, 50 cells per study). f Fura-2/AM Ca2+ imaging showing essentially abrogation of store-operated calcium entry (SOCE) activated by thapsigargin (1 μM) in PASMC from RyR2KO mice and statistical results of Ca2+ influx peak (n = 4 independent studies, 60 cells per study). Data are expressed as mean ± standard error. (*P < 0.05, using one-way ANOVA test or Student’s t test).