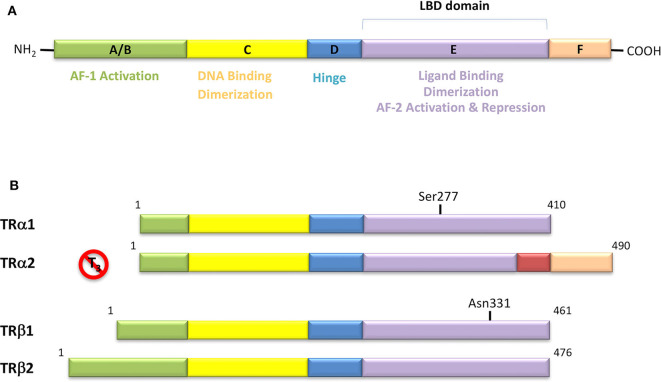
Figure 4.
(A) Domain organization of Thyroid Hormone Receptors (TRs) and functional regions. The TR is a member of the nuclear receptor (NR) protein family. The NRs share a common modular structure, which contains a variable A/B N-terminal region, a central conserved DNA-binding domain (DBD), a less conserved ligand-binding domain (LBD), and a linker region between the DBD and LBD. The N-terminal region harbors the activation function-1 (AF-1), which is independent of the LBD-ligand interaction. Within the LBD lies the activation function-2 (AF-2), with it referring to the recruitment of transcriptional activators in a ligand-dependent manner. (B) Schematic representation of the different isoforms of thyroid hormone receptor. There are two distinct TR genes (alpha and beta) that expressed as two differentially spliced isoforms (α1 and α2, and β1 and β2, respectively). TRα1 and TRβ1 are the predominant ligand binding forms in most tissues. Each receptor isoform conforms to the three-domain structure (NTD, DBD, and LBD) that is typical of NRs. The length of receptors is indicated just above the receptor diagrams. TRα2 cannot bind T3 because it contains a 122-amino acid carboxy terminus that replaces a region in TRα1 that is critical for TH binding.