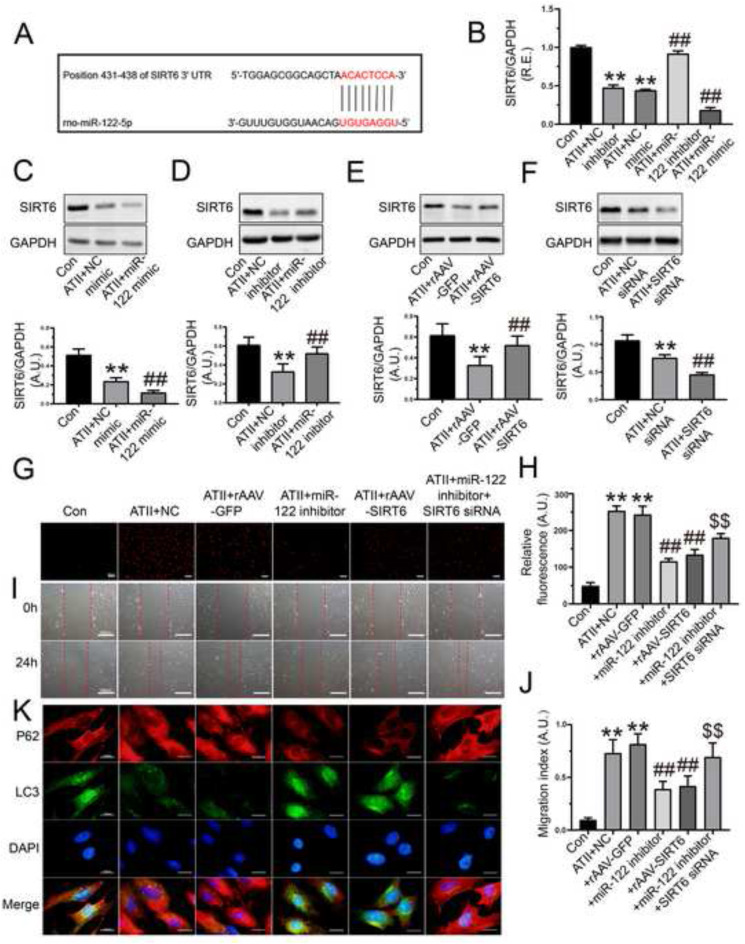
Fig. 4.
Treatment with miR-122-5p inhibitor prevented ATII-mediated promotion of oxidative stress, migration, and loss of autophagy in rat AFs. (A) The binding sites between miR-122-5p and 3′-UTR of SIRT6 mRNA. (B) Relative mRNA level of SIRT6 in rat AFs by qRT-PCR analysis. (C-D) Western blots to examine protein levels of SIRT6 in AFs after the transfection of miR-122-5p mimic and inhibitor, respectively. (E-F) Protein levels of SIRT6 in rat AFs after the overexpression and knockdown of SIRT6, respectively. (G-H) Representative dihydroethidium staining images and relative fluorescence of rat AFs. (I-J) In vitro wound healing to determine cellular migration of AFs at 0 h and 24 h. (K) Representative immunofluorescence images of p62 (red) and LC3 (green) to examine autophagic flux of rat AFs. GAPDH was used as an endogenous control. n = 3–4 for each group except for B where n = 6. **, P < 0.01 compared with control group; ##, P < 0.01 compared with ATII + NC or ATII + rAAV-GFP group; $$, P < 0.01 compared with ATII + miR-122 inhibitor group. A.U., arbitrary units; R.E., relative expression; ATII, angiotensin II; AFs, adventitial fibroblasts; NC, negative control; SIRT6, sirtuin 6.