Fig. 1.
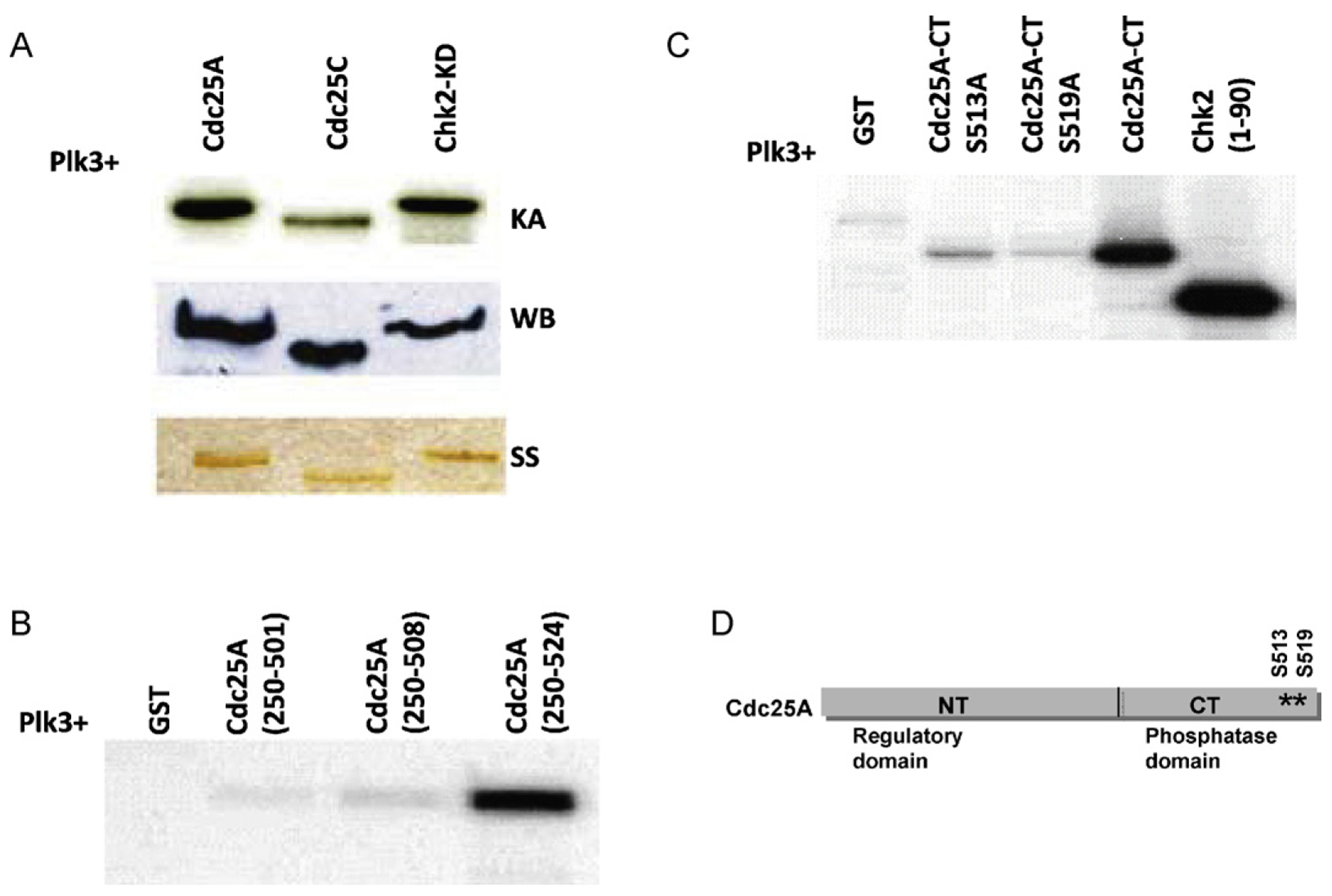
Plk3 phosphorylates Cdc25A on serines 513 and 519 in vitro. (A) GST fusion Plk3 protein was expressed in bacteria and used to phosphorylate either Cdc25A or known substrates of Plk3 (Cdc25C and Chk2) in a kinase assay (KA). The amounts of used substrates were checked by both Western blot (WB) and silver staining (SS). (B) Deletion mutants of Cdc25A show that the phosphorylation sites are within the last 16 amino acids of Cdc25A protein. (C) Mutation of S513 or S519 on Cdc25A abolishes Plk3 phosphorylation activity. (D) A schematic representation of Cdc25A protein showing the location of the two phosphorylated sites on the C-terminal part of the protein.
