Fig. 7.
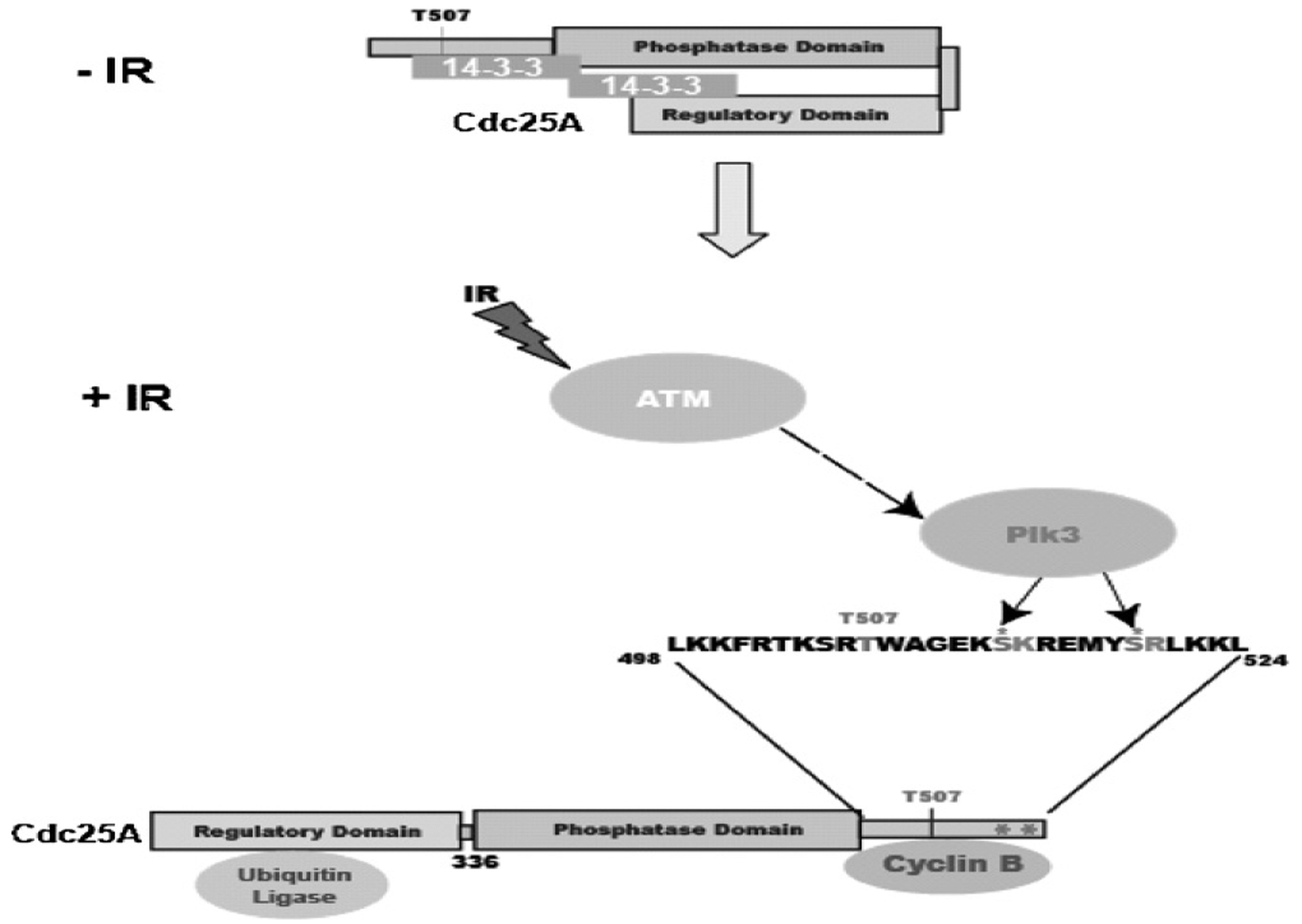
A model depicting the regulation of Cdc25A degradation by Plk3. Plk3 phosphorylates serines 513 and 519 which are adjacent to the cyclinB/cdk1 docking site (K514 and R520). This phosphorylation, together with Chk1 phosphorylation of T507 allows 14–3–3 binding and prevents cyclinB/cdk1 from binding to Cdc25A. 14–3–3 binding leads to an open conformation of Cdc25A that allows binding of the ubiquitin ligase and subsequent degradation of the protein.
