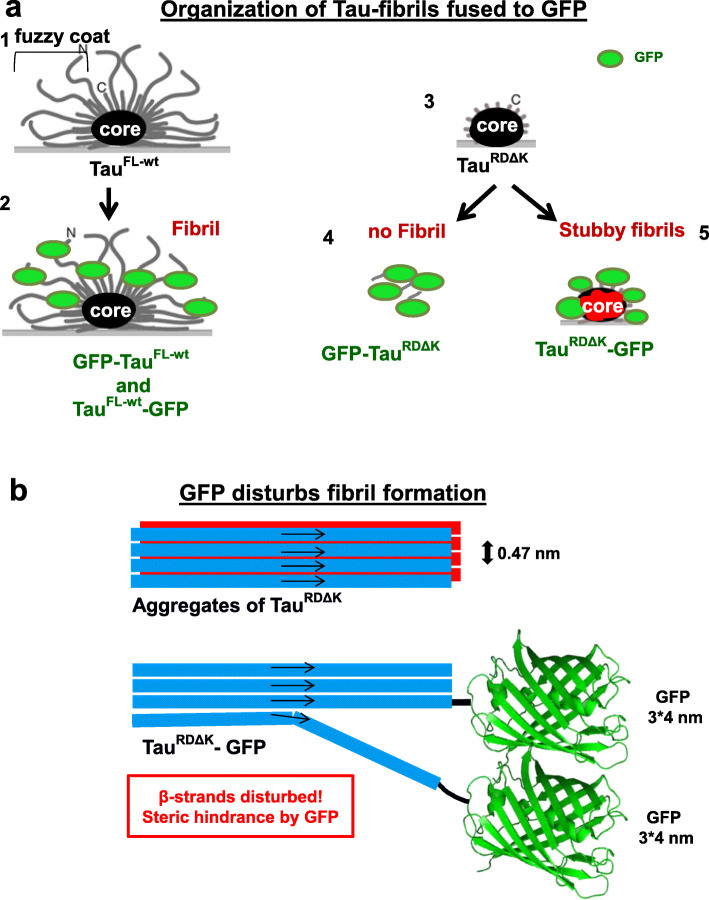
Fig. 7.
Models of organization of Tau fibrils fused to GFP. (a) Models of arrangement of GFP + Tau fusion constructs under aggregating conditions. The diagrams represent filaments lying on a support (grey line), viewed down the filament axis (black oval = core). (1, 2) TauFL-wt and GFP-TauFL-wt have a similar packing of their fibril cores (cross-β structure) with attached N- or C-terminal domains (1), plus GFP on either end (2). The PHF-like packing is possible because Tau is disordered and flexible, so that GFP molecules can be accommodated around the perimeter as a fuzzy “halo”. (3) TauRDΔK can form a PHF-like fibril core, but (4) GFP- TauRDΔK does not form any fibrils. (5) TauRDΔK-GFP may assemble into short aberrant fibrils, but the arrangement is not compatible with that of AD-like filaments and results in perturbed core (red distorted structure). The GFP creates an outside layer (analogous to the fuzzy coat of PHFs), but the packing is less dense and the elongation is strongly disturbed. (b) Diagram of a side view of a PHF-like filament, made up of two parallel β-sheets (red and blue) with antiparallel orientations (arrows). The axial separation of β-strands is 0.47 nm. Size comparison of β-strand and attached GFP. If the GFP were attached sufficiently far away from repeat domain (as in FLTau), the amyloid core could be formed, with GFP molecules accommodated around the perimeter. If the GFP is too close to the β-strand core (as in TauRD) this leads to steric hindrance which causes improper folding and disruption of the amyloid core