Abstract

Titanium oxide (TiO2) nano-/microparticles have been widely used in orthopedic and dental sciences because of their excellent mechanical properties, chemical stability, and ability to promote the osseointegration of implants. However, how the structure and crystallinity of TiO2 particles may affect their osteogenic activity remains elusive. Herein, we evaluated the osteogenic response to submicron amorphous, anatase, and rutile TiO2 particles with controlled size and morphology. First, the ability of TiO2 particles to precipitate apatite was assessed in an acellular medium by using a simulated body fluid (SBF). Three days after the addition to SBF, anatase and rutile TiO2 particles induced the precipitation of aggregates of nanoparticles with a platelike morphology, typical for biomimetic apatite. Conversely, amorphous TiO2 particles induced the precipitation of particles with poor Ca/P atomic ratio only after 14 days of exposure to SBF. Next, the osteogenic response to TiO2 particles was assessed in vitro by incubating MC3T3-E1 preosteoblasts with the particles. The viability and mineralization efficiency of osteoblastic cells were maintained in the presence of all the tested TiO2 particles despite the differences in the induction of apatite precipitation in SBF by TiO2 particles with different structures. Analysis of the particles’ surface charge and of the proteins adsorbed onto the particles from the culture media suggested that all the tested TiO2 particles acquired a similar biological identity in the culture media. We posited that this phenomenon attenuated potential differences in osteoblast response to amorphous, anatase, and rutile particles. Our study provides an important insight into the complex relationship between the physicochemical properties and function of TiO2 particles and sheds light on their safe use in medicine.
Introduction
Metallic titanium (Ti) and its alloys are one of the most used materials in orthopedic and oral implants because of their favorable properties including adequate mechanical strength, high corrosion resistance, and biocompatibility.1 The biocompatibility of Ti materials is assigned to the formation of a passivating oxide layer responsible for enhanced chemical stability and bioinertness. However, the passivating oxide layer creates a surface inert toward the fundamental biological events needed for osseointegration, which may lead to implant failure.2,3 In this scenario, tailoring the surface of implantable Ti materials has opened tremendous possibilities to trigger advanced cellular recognition and living tissue integration while maintaining the advantageous bulk properties of Ti.4−6 Especially, nanotopography modifications by surface coating with TiO2 micro-/nanoparticles have led to improved osseointegration by simply controlling the roughness of the implant surfaces. In fact, cells respond to surface features at the nanoscale and show preferential adhesion and proliferation on rough rather than flat surfaces.7−9 More interestingly, a topography-mediated bactericidal activity was obtained by coating Ti implants with TiO2 particles in the absence of antimicrobial agents.10,11 Such a synergistic effect of promoting osseointegration and preventing bacterial adhesion is an example of the advantages of surface-modified over uncoated metallic implants.12,13 Therefore, using nano-/micropatterned TiO2 leads to the possibility to explore a well-known biomaterial in new ways, triggering multiple functions only by optimizing the physicochemical features.14,15
Even though bulk Ti displays suitable biocompatibility, the cytotoxicity of TiO2 particles must be carefully assessed to support the use of Ti implants coated with TiO2 particles in medicine because the first contact with the host tissue occurs throughout the oxide layer. In fact, while nanosized (e.g., less than 100 nm in size) particles are internalized by bone cells and elicit oxidative stress and cell death,16 sub-micron-sized TiO2 particles exhibit low toxicity.17 Therefore, studies focused on the assessment of the impact of TiO2 particles on bone cells have mostly been conducted to improve the performance of bone-healing biomaterials18 and hamper the release of debris from implants, which may interact with adjacent tissues and cells.19
Most of the approaches adopted to build Ti-based biomaterials have led to multiple crystal phases of TiO2, namely, anatase,20 rutile,21 and brookite.22 Although the structure–function relationship is fundamental to the cell response to particles,23−25 the crystallinity of TiO2 is often neglected in biomaterial design, and its role on cell response is still a matter of debate. In fact, some investigations have been conducted using TiO2 films;20,26 however, the influence of surface roughness and hydrophilicity on cell adhesion makes it difficult to identify the effects of the crystalline phase. TiO2 particles have also been used to this end,27 but differences in terms of size, morphology, and colloidal stability have led to contradictory findings. Moreover, the fabrication of TiO2 particles of a well-defined and pure structure is still an unresolved challenge. Given the ongoing need of improving bone regenerative therapies, such a delineation is fundamental to the rational design and optimization of Ti-based implants. Additionally, humans are constantly exposed to TiO2 particles as they are common additives in food and personal care products.28 Thus, gathering more information about this material would benefit several research fields.
Along with the fabrication of TiO2 particles of well-defined and pure crystallinity, the assessment of the relationship between the physical properties and function of TiO2 particles is hampered by an additional challenge related to a phenomenon that arises when particles of both nano and micron size enter the biological milieu. Before interacting with the cells, the particles come into contact with biological fluids, which contain ions, proteins, and other types of biomacromolecules. These components promptly adsorb onto the surface of the particles, giving rise to the so-called particle–corona complexes, which differ from the bare particles. The corona confers a new identity to the particles, often referred to as “biological identity” to distinguish it from the identity conferred to bare particles by the physicochemical properties or “synthetic identity”. Hence, the “biological identity” is what cells really “see” of the particles.29 Recent studies have proven that the “biological identity” directly mediates the relationship between the synthetic identity and cellular fate of the particles. Thus, the assessment of the complex relationship among the synthetic identity, biological identity, and function of TiO2 particles would be pivotal for their safe use in medicine as well as in nutrition and cosmetic sciences.
In view of these considerations, herein amorphous, anatase, and rutile TiO2 particles with controlled morphology were fabricated, characterized, and applied to understand how the particles’ structure and crystallinity impact their ability to induce apatite precipitation. The ability of TiO2 particles to precipitate apatite was assessed in both the absence and the presence of osteogenic cells by adding the particles to an acellular medium, that is, simulated body fluid (SBF), and MC3T3-E1 preosteoblasts, respectively. We found that the particle structure tailored the type of mineral precipitated in SBF. Conversely, the effects of the particles on the viability and mineralization efficiency of MC3T3-E1 cells were independent of their structure and crystallinity. Analysis of the particles’ surface charge and the proteins adsorbed onto the particles from the culture media suggested that all the tested TiO2 particles acquired a similar biological identity in the culture media. We posited that this phenomenon made osteoblast response to TiO2 particles independent of their physicochemical properties. Our study provides an important insight into the complex relationship between the physicochemical properties and function of TiO2 particles and sheds light on their safe use in medicine.
Experimental Section
Synthesis of TiO2 Particles
Synthesis of TiO2 particles was performed through a modified sol–gel method described elsewhere.30 Briefly, hexadecylamine (7.95 g, 90%, Sigma-Aldrich) was dissolved in ethanol (800 mL, 99.7%, Merck), followed by the addition of 0.1 mol L–1 of a KCl aqueous solution (3.20 mL, 99%, Fluka). Sequentially, titanium(IV) isopropoxide (18.10 mL, 97%, Sigma-Aldrich) was added to this solution under vigorous stirring at room temperature. The milky white precursor suspension was kept static for 18 h and then centrifuged at 3000 rpm for 2 min and washed with ethanol. The washing cycles were repeated three times and dried in air at room temperature. Postsynthetic heat treatment was employed to attain anatase and rutile TiO2 polymorphs. Thus, the particles were taken inside a furnace at 600 or 900 °C for 5 min and then cooled down at room temperature.31
Characterization of TiO2 Particles
The morphology of gold-coated particles was investigated by scanning electron microscopy (SEM) using a Zeiss-EVO 50 microscope under a 20 kV accelerating voltage. The X-ray diffraction patterns were acquired using a Bruker-AXS D5005 diffractometer using Cu Kα radiation. The diffraction peaks were indexed based on the databank of the Joint Committee on Powder Diffraction Standard (JPDCS). The hydrodynamic diameter and zeta potential (ζ) of the particles were measured using Zetasizer Nano ZS (Malvern Instruments). For this, the particles were dispersed either in phosphate-buffered solution (PBS, pH 7.4, 10 mM) or in a minimum essential medium (α-MEM, Gibco) supplemented or not with 10% fetal bovine serum (FBS) and 1% (v/v) penicillin/streptomycin. The measurements were carried out in triplicate at room temperature.
Protein Corona Evaluation and Sodium Dodecylsulfate-Polyacrylamide Gel Electrophoresis
The particles were incubated for 2 h in α-MEM, supplemented with 10% FBS. After centrifugation, the nanoparticle–protein complexes were washed three times with 10 mM PBS (pH 7.4) in order to remove the excess nonadsorbed proteins. The nature of the proteins adsorbed at the surface of the particle was investigated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For this, the nanoparticle–protein corona pellet was resuspended in a buffer (50 mM Tris-HCl, pH 6.8, 2% (w/v) SDS, 10% glycerol, and 357 mM 2-mercaptoethanol), followed by boiling for 5 min at 100 °C. Gel electrophoresis was performed at 120 V, until the proteins reached near the end of the gel. Then, the SDS-PAGE gels were silver-stained. The serum-supplemented cell culture medium (10% FBS) was used as a standard for comparison.
In Vitro Biomimetic Mineralization Ability
The TiO2 particles were suspended in an SBF at 37 °C for 3, 7, and 14 days to evaluate their ability to induce biomimetic precipitation of apatite in a physiological environment. An SBF resembles the ionic composition and pH of the blood plasma. The procedure used to prepare an SBF followed the International Standard Organization (ISO 23317) and is described elsewhere.32 Briefly, 2.5 mmol L–1 of Ca2+, 142 mmol L–1 of Na+, 4.2 mmol L–1 of HCO3–, 5 mmol L–1 of K+, 1.5 mmol L–1 of Mg2+, 147.8 mmol L–1 of Cl–, 1 mmol L–1 of HPO42–, and 0.5 mmol L–1 of SO42– were dissolved in ultrapure water. The pH was adjusted to 7.4 with tris-hydroxymethyl amine methane (Tris) and 1 mol L–1 of HCl aqueous solution. The Ca/P atomic ratios were determined by energy-dispersive X-ray (EDX) spectrometry coupled to a scanning electron microscope (Zeiss-EVO 50). The samples were coated with graphite before the analysis.
In Vitro Osteogenic Response to TiO2 Particles
The osteoblastic lineage cells MC3T3-E1 (American Type Culture Collection—ATCC) were cultivated in α-MEM, Gibco, supplemented with 10% FBS and 1% (v/v) penicillin/streptomycin. This is a lineage with an osteoblastic phenotype that undergoes proliferation–differentiation, leading to mineralized matrix formation under osteogenic conditions. The osteogenic medium was achieved by the addition of ascorbic acid and β-glycerophosphate. Briefly, the cells were seeded on 24-well plates at a density of 2 × 104 cells per well and incubated in air at 37 °C and 5% CO2. The plated cells were then allowed to attach to the polystyrene well bottoms for 24 h, followed by the replacement of the medium for a 1 μg mL–1 suspension of the particles dispersed in the culture medium. The culture medium was changed every 2 days keeping the concentration of the particles constant. Cell viability was determined by the classic 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay after 7, 14, and 21 days of culture using the protocol described by Mosmann.33 Cell viability was expressed as the percentage of the average of three experiments as compared to the control (Ct) without treatment for each day of culture (100%).
Alkaline Phosphatase Activity and Mineralized Nodule Quantification
Alkaline phosphatase (ALP) activity was determined by quantifying its action on the substrate p-nitrophenyl phosphate in the plasma membrane fraction extracted from cells after 7, 14, and 21 days of culture according to the protocol described elsewhere.34 The enzymatic activity was normalized by the total protein content, which was estimated in the presence of 2% (w/v) SDS using bovine serum albumin as a standard. After culturing for 21 days, the mineralized extracellular matrix was quantified by Alizarin Red staining.35 To this end, the wells were rinsed with PBS to remove precipitated TiO2 particles.
Cell Morphology
SEM and confocal microscopy were applied to investigate cell morphology. The attached cells were stained with acridine orange (5 μg mL–1) and then visualized using a confocal laser scanning microscope (Leica TCS SP5 microscope). This fluorophore binds to the osteoblast nucleic acids (RNA in red and DNA in green), providing a contrasted image. To visualize DNA, the sample was excited with the 488 nm line of an argon laser, and emission was collected between 499 and 541 nm. RNA was visualized by exciting the sample with the 458 nm line of an argon laser, and emission was collected between 642 and 682 nm. For the SEM images, the cells were fixed in 1.5% glutaraldehyde at 4 °C for 12 h, dehydrated through a series of ethanol concentrations (20, 50, 70, 80, 90, and 100%), and contrasted with osmium tetroxide. After coating with gold, the samples were then observed on a Zeiss-EVO 50 SEM microscope under a 20 kV accelerating voltage. EDX analysis was carried out in carbon-coated samples using the same equipment.
Results and Discussion
Characterization of TiO2 Particles
The X-ray diffraction patterns confirmed the synthesis of well-defined and amorphous, anatase, and rutile TiO2 particles (Figure 1A). The absence of peaks in the diffractogram of the as-prepared sample confirmed its amorphous nature. Reflux in ethanol was applied to the as-prepared product to attain amorphous particles free of stabilizing agents. The particles annealed at 600 and 900 °C displayed typical diffraction patterns from anatase and rutile, respectively (Figure 1A). To confirm surfactant elimination, the particles were further characterized by ζ measurements (Figure 1B). The decrease of ζ values toward negative values after annealing can be assigned to the complete removal of the cationic surfactant hexadecylamine from the particles.
Figure 1.
(a) X-ray diffraction patterns of the as-prepared TiO2 particles after reflux in ethanol (amorphous), annealed at 600 °C (anatase) and 900 °C (rutile). The blue and red vertical lines correspond to the diffraction pattern of anatase (JCPDS 21-1272) and rutile (JCPDS 21-1276), respectively. (b) ζ potential vs pH acquired for aqueous dispersions containing the as-prepared (▼), amorphous (▲), anatase (■), and rutile (●) particles.
SEM images showed that all the fabricated TiO2 particles had a spherical shape and were uniform in size and morphology (Figure 2a,c,e). Dynamic light scattering (DLS) analysis revealed that the particles were monodisperse in size with an average hydrodynamic radius close to 600 nm (Figure 2b,d,f). The ability to access to amorphous, anatase, and rutile TiO2 particles that are uniform in size and morphology is crucial to assess the role played by structure and crystallinity on the function of these particles, excluding possible effects driven by other physicochemical parameters.
Figure 2.
SEM images (a,c,e) and DLS hydrodynamic diameter (D) distribution in water (b,d,f) of amorphous (a,b), anatase (c,d), and rutile (e,f) TiO2 particles. Scale bars correspond to 1 μm.
Reactivity of TiO2 Particles toward Apatite Precipitation in a Biomimetic Environment
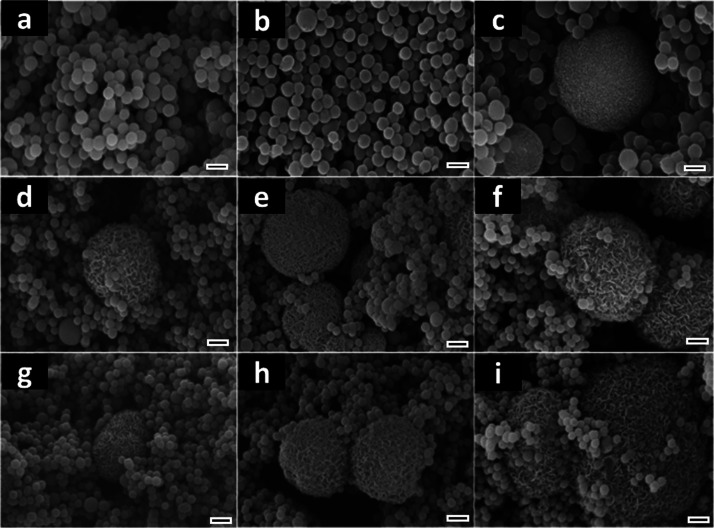
The formation of a superficial bonelike apatite layer after coming in contact with the body fluid is regarded as one of the main features of bioactive biomaterials.36 This mineral layer decreases the surface free energy at the biomaterial/bone interface and favors the binding between the implant and the living tissue.37 This is, therefore, an essential requirement to hamper the formation of a fibrous tissue encapsulating and isolating the implanted material from the host bone, which would lead to implant failure.38 Given the paramount importance of such aspect, the immersion of biomaterials in SBF, which simulates the blood plasma in terms of pH, ionic strength, and composition, is a standard assay to investigate their ability to form apatite in the absence of cells.32 Thus, in order to better understand the implications of the TiO2 particle’s structure on apatite nucleation and growth in a physiological environment, the particles were soaked in SBF for periods of 3, 7, and 14 days. Microparticles made of aggregates of nanoparticles with a platelike morphology typical for biomimetic apatite were formed in the anatase and rutile samples, after 3 days of SBF exposure (Figure 3d,g). The amount and size of the microparticles increased with the exposure time, indicating an augment in phosphate deposition (Figure 3e,f,h,i). Energy-dispersive spectrometry (EDS) analysis showed an initial Ca/P atomic ratio of 1.9 for anatase and rutile after 3 days in SBF, suggesting the formation of Ca2+-rich amorphous phosphate (Table 1). The Ca/P ratio decreases to 1.7 after 14 days of SBF exposure, suggesting the conversion of amorphous phosphate into apatite. It is worth noting that no significant difference in terms of calcium phosphate formation was detected between anatase and rutile phases. Conversely, amorphous TiO2 displayed a poor ability to induce apatite precipitation as revealed by the absence of calcium phosphate aggregates after 7 days of SBF exposure (Figure 3a,b) and by the low Ca/P ratios typical of Ca2+-poor phosphate (Table 1).39
Figure 3.
SEM images of amorphous (a–c), anatase (d–f), and rutile (g–i) TiO2 particles exposed to SBF for 3 (a,d,g), 7 (b,e,h), and 14 (c,f,i) days. Scale bars correspond to 1 μm.
Table 1. Ca/P Atomic Ratios Determined for TiO2 Particles after Exposure to SBF for 3, 7, and 14 Daysa.
| Ca/P |
|||
|---|---|---|---|
| day | amorphous | anatase | rutile |
| 3 | 1.4 | 1.9 | 1.9 |
| 7 | 1.2 | 1.8 | 1.7 |
| 14 | 1.0 | 1.7 | 1.7 |
Ca2+ and PO43– ions were attracted from the SBF toward the surface of the TiO2 particles where they reached the supersaturation concentration of calcium phosphate and led to mineral precipitation.
The precipitation of apatite on TiO2 in a biological medium is related to the formation/hydrolysis of surface hydroxyl groups, which, in turn, give rise to negatively charged sites. Thus, Ca2+ ions are electrostatically attracted from the medium to the Ti surface, leading to the formation of nucleation spots. In the sequence, the migration of PO43– ions triggers the precipitation of amorphous calcium phosphate, which is transformed into apatite nuclei by redissolution and reprecipitation steps.40 The growth of apatite nuclei is fed by the continuous consumption of Ca2+ and PO43– ions from the medium. Nonetheless, only certain types of OH groups in a specific structural arrangement are effective in inducing the epitaxy of apatite growth.41 Consequently, because of the lack of spatial orientation, amorphous TiO2 usually displays poor bioactivity as observed by the late apatite precipitation compared to the anatase and rutile TiO2 particles (Figure 3). Similarly, the immersion of TiO2 films into the SBF has revealed that the number of OH groups is not crucial to induce apatite formation, whereas the presence of a crystalline phase is fundamental regardless of anatase, rutile, or a mixture of both.42,43
In Vitro Osteogenic Response
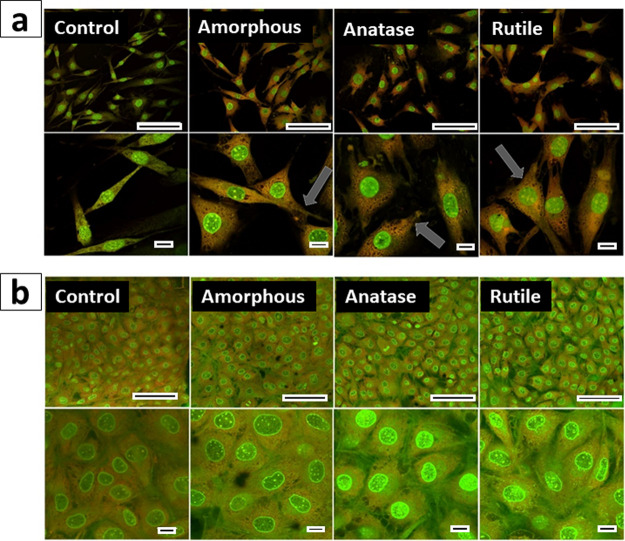
It is well established that a Ti surface with a nanoscale topography favors osteoblast differentiation by modulating different signaling pathways.44 However, none of these studies have taken into account neither the formation of a TiO2 layer on the surface of implants nor the possible role of structure and crystallinity on osteoblast response. To investigate how TiO2 structure and crystallinity may affect bone formation, we studied the impact of TiO2 particles on osteoblast adhesion, proliferation, and metabolic activity. To this end, MC3T3-E1 cells were cultured for up to 21 days in mineralization medium in the presence of TiO2 particles. Cells cultured in the absence of particles were used as a control to normalize the data on cell growth and mineralization. First, we evaluated how TiO2 particles affect osteoblast adhesion and proliferation by means of confocal microscopy (Figure 4). The high cell density attested the proliferation. The cells spread and exhibited a polygonal shape and larger area after 24 h of culture in the presence of particles, whereas control cells remained with a fusiform morphology, suggesting an early attachment stage (Figure 4a).45 Filopodia extending from the cells’ base toward the coverslip surface and other cells (Figure 4a, gray arrows) suggested anchoring processes and intercellular connections.46 Overall, cell morphology was similar in the presence of both amorphous and crystalline particles. After 7 days of culture, the cells enlarged and flattened, displaying a polygonal shape typical of osteoblasts (Figure 4b).47 These results confirm that osteoblasts attached, spread, and proliferated regardless of the particles’ structure and crystallinity.
Figure 4.
Confocal images of MC3T3-E1 cells cultured for 24 h (a) and 7 days (b) in the absence of particles (control) and in the presence of amorphous, anatase, and rutile TiO2 particles. The gray arrows indicate filopodia extending from the cells’ base toward the coverslip surface. Scale bars correspond to 50 and 10 mm in the upper and lower panels, respectively.
Cell morphology was also investigated by means of SEM (Figure 5). After 14 and 21 days of culturing, the cells attached and spread, reaching confluence regardless of the presence of TiO2 particles. Moreover, the cells exhibited a flattened and elongated morphology and grew with parallel orientation forming multilayers typical of mature osteoblasts.48 Submicron size globular features were observed on the cell surface (Figure 5, white arrows). The EDS analysis did not evidence the presence of Ti on the cell surface (red arrow, Figure 6); thus, the globular features were not identified as TiO2 particles lying external to or immediately beneath the plasma membrane. The EDS spectra showed the presence of Ti only in small dots distributed throughout the cell-free regions of the culture plate, suggesting that the particles were not internalized by the cells as already described for submicron TiO2 (blue arrow, Figure 6).49 Because the globular features were present on the surface of osteoblasts differentiated to a mineralizing phenotype, we posited that they were matrix vesicles (MVs), a special class of extracellular vesicles (EVs) mediating physiological and ectopic biomineralization processes.50 These vesicles were found in appreciable amount after 14 days for the cells cultured in the presence of TiO2 particles, whereas they were visible only after 21 days for the control cells. We cannot rule out that the globular features observed on the surface of differentiated osteoblasts belong to other classes of EVs (i.e., exosomes and microvesicles) different from MVs. The release of microvesicles related to the secretion of certain proteins from MC3T3-E1 cells under mineralization conditions has also been described.48,51
Figure 5.
SEM images of MC3T3-E1 cells cultured for 14 and 21 days in the absence of particles (a–d) or in the presence of amorphous (e–h), anatase (i–l), and rutile (m–p) TiO2 particles. The arrows indicate the presence of submicron globular features on the cell surface. We posited that the globular features were MVs. Scale bars correspond to 10 μm in the lower magnification images and 1 μm in the enlarged images.
Figure 6.

EDS analysis of MC3T3-E1 cells cultured for 14 days in the presence of anatase particles. The red and blue arrows indicate a cell and a cell-free region, respectively, of the culture plate, where the EDS spectra were recorded.
Cell viability was investigated by the MTT assay (Figure 7). The presence of the particles did not significantly alter the MC3T3-E1 proliferation rate, thus suggesting that they were not toxic to osteoblasts even after long culturing periods (14 and 21 days) (Figure 7a). Additionally, we found that the particles had no influence on the activity of ALP, an enzyme that generates inorganic phosphate during bone mineralization and one of the most important markers of the early stage of osteoblast differentiation (Figure 7b). Moreover, the maximum of ALP activity observed at the 14th day of culture is typical of, and compatible with, active osteoblasts.34 In agreement with these results, we observed no differences in the amount of calcified nodules secreted by the cells after 21 days of culturing (Figure 7c). Previous studies reported a decrease in the proliferation of MC3T3-E1 cells cultured in the presence of TiO2 particles at the same concentration range used in the present study (μg/mL).27 However, besides concentration, cell viability is dependent on particle morphology,52 size,53 and structure.54 For instance, while submicrometric anatase particles were not toxic to osteoblasts, anatase TiO2-faceted nanocrystals with high {001} percentages in relation to {101} significantly reduced cell viabilities and induced a series of toxicological responses driven by an oxidative stress mechanism.55 Although meaningful efforts have been made in this regard, the reports are still contradictory because of the differences in the physicochemical properties of the particles used. Moreover, cell lineages display different susceptibilities to particles.56 By using a nonthermal approach to convert amorphous TiO2 into anatase and rutile, Rodriguez-Contreras et al. have shown that osteogenic cells performed similarly after coming in contact with both amorphous and crystalline TiO2 particles, in agreement with our findings.57
Figure 7.
In vitro osteogenic response of MC3T3-E1 cells to TiO2 particles: (a) % of cell viability vs control measured by the MTT assay after 12 h (white bar), 24 h (light gray bar), 72 h (dark gray bar), 7 days (hashed light gray bar), 14 days (hashed light gray bar), and 21 days (hashed dark gray bar) of culture; (b) activity of ALP in the osteoblasts’ membrane fraction after 7 (white bar), 14 (light gray bar), and 21 days (dark gray bar) of culture and (c) quantification of mineralized nodules formed in the wells after 21 days of culture. No statistically significant differences were observed in the cell viability, ALP activity, and mineralized nodule formation among amorphous, anatase, and rutile TiO2 particles and the control.
TiO2 Particle Behavior in Biological Environment
Although most of the studies on the biological performance of particles have been conducted by considering the materials’ intrinsic physicochemical properties, also referred to as “synthetic identity”, the latter is not what cells really “see” of the particles.29 In fact, particles are coated by a corona of ions, proteins, and other types of biomacromolecules as soon as they enter the biological milieu and acquire a new identity, referred to as “biological identity”.58,59 Recently, studies have proven that the “biological identity” directly mediates the cellular fate of the particles.59−62 Therefore, we assessed if the pattern of proteins, also referred to as “protein corona”, adsorbed onto TiO2 particles in the cell culture medium changed with the structure and crystallinity of the particles in order to better understand their interaction with cells.
TiO2 particles were added to the cell culture medium supplemented or not with FBS, a complex solution composed of about 3700 proteins with albumin as the protein with the highest concentration.63 Control particles were added to PBS. The particles displayed a mean hydrodynamic radius of ∼600 nm in both PBS and serum-supplemented cell culture medium, whereas they displayed a hydrodynamic radius of ∼900 nm in serum-free medium, suggesting particle aggregation regardless of structure and crystallinity (Figure 8a). Particle aggregation in serum-free cell medium has been described, and it is mostly related to the high ionic strength because of the dissolved salts that screen the particles’ surface charges.64 However, if serum is present, the particles are coated and sterically stabilized by serum proteins.65 ζ potential measurements were performed to validate the involvement of serum proteins in the identity acquired by the particles in the culture medium. The negative ζ potential (∼−30 mV) observed for TiO2 particles in PBS is related to the deprotonation of negatively charged surface hydroxyl groups. Interestingly, when immersed in the culture medium, the particles displayed a similar ξ value (∼−10 mV) regardless of the presence of serum (Figure 8b). Such a drop in ξ value is most likely due to the interplay between the selective binding of ions such as Ca2+ and PO43–, ionic strength, and adsorption of cell medium constituents such as small molecules and proteins.19 Therefore, there is evidence that the colloidal stability displayed by TiO2 particles in serum-supplemented medium mostly arises from the adsorption of a protein corona on the particles’ surface. In order to investigate the nature of the protein corona formed on TiO2 particles, SDS-PAGE was carried out (Figure 8c). We found that all the TiO2 particles displayed a protein corona mostly formed by proteins of approximately 67 kDa. Other proteins with higher and lower MW also adsorbed onto the particles’ surface. By proteomic analysis, Ribeiro et al. found that the main proteins adsorbed onto anatase particles incubated with 10% FBS-supplemented culture medium were albumin and glycoproteins (including alpha 2HS glycoprotein).66 Although further studies are warranted to fully characterize the composition of the protein corona adsorbed onto anatase, rutile, and amorphous TiO2 particles, our results strongly suggest that the adsorption of ions and proteins of the culture medium may have conferred similar identity to the particles regardless of their structure and crystallinity. This phenomenon, in turn, may be responsible for the absence of differences in viability and mineralization efficiency of osteoblasts treated with anatase, rutile, and amorphous TiO2 particles. Although the mechanism of TiO2 particle cytotoxicity is still a matter of debate, it is known that the particles’ surface charge changes the local environment and influences their cellular interactions and intracellular fate.17,67 In fact, it has been shown that the surface charge is related to the type of proteins adsorbed on the surface of particles in a biological environment and dictates the interaction with lipid membranes.68,69 Besides that, other interactions, namely, London dispersion and hydrogen bonding, have also been described to play a part in the particles’ biological response.70 The size and morphology have also been found to drive the biological performance of particles both directly and indirectly by affecting the formation of a protein corona on particles.71 Therefore, as the particles investigated herein differ from each other only in terms of structure and crystallinity, we posited that upon contact with the cell culture medium, the resulting particle/ion/protein complexes had a similar biological identity as evidenced by the ξ potential and SDS-PAGE analyses. This finding may also explain why the ability of inducing apatite precipitation was different among anatase, rutile, and amorphous in SBF, whereas no difference was observed in cell culture. As the cells interact with the whole complexes rather than the bare particles, it would be reasonable to correlate osteoblast responses to the different TiO2 particles to their similar biological identity.29 Taken together, these results strength the paradigm that the biological identity acquired by particles in the biological milieu has a predominant role on their biological performance and may mask the possible effects arising from the physicochemical properties, that is, the synthetic identity. Indeed, similar results have already been reported in the literature:72 regardless of the initial synthetic identity, the adsorption of serum proteins leads to particles with similar biological identity and consequently biological performance. Ribeiro et al. showed that the corona of ions and proteins acted as a camouflage for anatase particles, and it affected the interactions of the particles with osteoblasts and their ability to attract Ca2+ and PO43– from the culture medium.19,66 However, it is worth pointing out that the particle structure and crystallinity may lead to subtle differences in the nature and conformation of proteins adsorbed on TiO2 particles; thus, a direct effect of these properties, as well as other features of the particles’ synthetic identity, on their biological performance cannot be completely ruled out.73,74 Further studies are warranted to characterize the nature and conformation of the proteins adsorbed on TiO2 particles in a physiological environment.
Figure 8.
Hydrodynamic diameters (a) and ζ values (b) of TiO2 particles in PBS (black bars), FBS-free culture medium α-MEM (gray bars) and 10% FBS-supplemented culture medium α-MEM (white bars). No statistically significant difference was observed when comparing the size and ζ of the TiO2 particles in the same medium. SDS-PAGE (c) of the proteins extracted from the surface of the particles after immersion into 10% FBS-supplemented culture medium α-MEM compared to 10% FBS-supplemented culture medium α-MEM and the pattern of molecular weight (MW) (kDa).
Conclusions
Herein, amorphous, anatase, and rutile TiO2 particles with controlled morphology were fabricated, characterized, and applied to understand how the particles’ structure and crystallinity impact their ability to induce apatite precipitation. We found that anatase and rutile TiO2 particles induced apatite precipitation differently from amorphous TiO2 particles after coming in contact with SBF. Conversely, MC3T3-E1 preosteoblasts adhered, proliferated, and mineralized in the presence of TiO2 particles regardless of their structure and crystallinity. These results may be explained by the fact that a similar corona of ions and proteins formed onto the TiO2 particles that are in contact with the cell culture medium irrespective of their structure and crystallinity. This “biocamouflage” of the particles’ physicochemical properties would strengthen the predominant role of biological identity on the osteogenic response to TiO2 particles. Overall, our results reinforce the concept that medium components should be considered during both in vitro and in vivo studies as they dictate the biological identity of the materials and, in turn, what the cells really “see” of them. Further efforts are warranted to understand the possible impacts of adsorption onto different types of TiO2 particles on protein composition and conformation. Our study provides an important insight into the complex relationship among the synthetic identity, biological identity, and biological performance of TiO2 particles and sheds light on their safe use in medicine.
Acknowledgments
C.B.T. thanks FAPESP (2014/24249-0) for the scholarships. C.R.F. and Maytê Bolean thank CNPq and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil, Finance Code 001, #88887.320304/2019-00) for the scholarships, respectively. A.P.R. and P.C. thank FAPESP for research grants (2017/08892- and 2016/21236-0). P.C. and A.P.R. are CNPq researchers.
The authors declare no competing financial interest.
References
- Long M.; Rack H. J. Titanium Alloys in Total Joint Replacement — a Materials Science Perspective. Biomaterials 1998, 19, 1621–1639. 10.1016/s0142-9612(97)00146-4. [DOI] [PubMed] [Google Scholar]
- Albrektsson T.; Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M.; Lausmaa J.; Hirsch J.-M.; Thomsen P. Surface Analysis of Failed Oral Titanium Implants. J. Biomed. Mater. Res. 1999, 48, 559–568. . [DOI] [PubMed] [Google Scholar]
- Jiang N.; Guo Z.; Sun D.; Li Y.; Yang Y.; Chen C.; Zhang L.; Zhu S. Promoting Osseointegration of Ti Implants through Micro/Nanoscaled Hierarchical Ti Phosphate/Ti Oxide Hybrid Coating. ACS Nano 2018, 12, 7883–7891. 10.1021/acsnano.8b02227. [DOI] [PubMed] [Google Scholar]
- Tovani C. B.; Faria A. N.; Ciancaglini P.; Ramos A. P. Collagen-Supported CaCO3 Cylindrical Particles Enhance Ti Bioactivity. Surf. Coat. Technol. 2019, 358, 858–864. 10.1016/j.surfcoat.2018.11.071. [DOI] [Google Scholar]
- Cruz M. A. E.; Zanatta M. B. T.; da Veiga M. A. M. S.; Ciancaglini P.; Ramos A. P. Lipid-mediated growth of SrCO3/CaCO3 hybrid films as bioactive coatings for Ti surfaces. Mater. Sci. Eng., C 2019, 99, 762–769. 10.1016/j.msec.2019.02.023. [DOI] [PubMed] [Google Scholar]
- Gui N.; Xu W.; Abraham A. N.; Shukla R.; Qian M. Osteoblast Responses to Titanium-Coated Subcellular Scaled Microgrooves. ACS Appl. Bio Mater. 2019, 2, 2405–2413. 10.1021/acsabm.9b00094. [DOI] [PubMed] [Google Scholar]
- Gittens R. A.; Olivares-navarrete R.; Cheng A.; Anderson D. M.; Mclachlan T.; Stephan I.; Geis-gerstorfer J.; Sandhage K. H.; Fedorov A. G.; Rupp F.; Boyan B. D.; Tannenbaum R.; Schwartz Z. The roles of titanium surface micro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 2013, 9, 6268–6277. 10.1016/j.actbio.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghihi S.; Zhilyaev A. P.; Szpunar J. A.; Azari F.; Vali H.; Tabrizian M. Nanostructuring of a Titanium Material by High-Pressure Torsion Improves Pre-Osteoblast Attachment. Adv. Mater. 2007, 19, 1069–1073. 10.1002/adma.200602276. [DOI] [Google Scholar]
- Huo K.; Zhang X.; Wang H.; Zhao L.; Liu X.; Chu P. K. Osteogenic Activity and Antibacterial Effects on Titanium Surfaces Modified with Zn-Incorporated Nanotube Arrays. Biomaterials 2013, 34, 3467–3478. 10.1016/j.biomaterials.2013.01.071. [DOI] [PubMed] [Google Scholar]
- Elsaka S. E.; Hamouda I. M.; Swain M. V. Titanium Dioxide Nanoparticles Addition to a Conventional Glass-Ionomer Restorative: Influence on Physical and Antibacterial Properties. J. Dent. 2011, 39, 589–598. 10.1016/j.jdent.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Ferraris S.; Spriano S. Antibacterial Titanium Surfaces for Medical Implants. Mater. Sci. Eng., C 2016, 61, 965–978. 10.1016/j.msec.2015.12.062. [DOI] [PubMed] [Google Scholar]
- Luo Q.; Huang Y.; Zha G.; Chen Y.; Deng X.; Zhang K.; Zhu W.; Zhao S.; Li X. Topography-Dependent Antibacterial, Osteogenic and Anti-Aging Properties of Pure Titanium. J. Mater. Chem. B 2015, 3, 784–795. 10.1039/c4tb01556h. [DOI] [PubMed] [Google Scholar]
- Spriano S.; Yamaguchi S.; Baino F.; Ferraris S. A Critical Review of Multifunctional Titanium Surfaces: New Frontiers for Improving Osseointegration and Host Response, Avoiding Bacteria Contamination. Acta Biomater. 2018, 79, 1–22. 10.1016/j.actbio.2018.08.013. [DOI] [PubMed] [Google Scholar]
- Zwahr C.; Helbig R.; Werner C.; Lasagni A. F. Fabrication of Multifunctional Titanium Surfaces by Producing Hierarchical Surface Patterns Using Laser Based Ablation Methods. Sci. Rep. 2019, 9, 6721. 10.1038/s41598-019-43055-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouiller B.; Reliene R.; Westbrook A.; Solaimani P.; Schiestl R. H. Titanium Dioxide Nanoparticles Induce DNA Damage and Genetic Instability in Vivo in Mice. Cancer Res. 2009, 69, 8784–8789. 10.1158/0008-5472.can-09-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setyawati M. I.; Tay C. Y.; Chia S. L.; Goh S. L.; Fang W.; Neo M. J.; Chong H. C.; Tan S. M.; Loo S. C. J.; Ng K. W.; et al. Titanium Dioxide Nanomaterials Cause Endothelial Cell Leakiness by Disrupting the Homophilic Interaction of VE-Cadherin. Nat. Commun. 2013, 4, 1673. 10.1038/ncomms2655. [DOI] [PubMed] [Google Scholar]
- Zhang B.; Pei X.; Zhou C.; Fan Y.; Jiang Q.; Ronca A.; D’Amora U.; Chen Y.; Li H.; Sun Y.; Zhang X. The Biomimetic Design and 3D Printing of Customized Mechanical Properties Porous Ti6Al4V Scaffold for Load-Bearing Bone Reconstruction. Mater. Des. 2018, 152, 30–39. 10.1016/j.matdes.2018.04.065. [DOI] [Google Scholar]
- Ribeiro A. R.; Mukherjee A.; Hu X.; Shafien S.; Ghodsi R.; He K.; Gemini-Piperni S.; Wang C.; Klie R. F.; Shokuhfar T.; Shahbazian-Yassar R.; Borojevic R.; Rocha L. A.; Granjeiro J. M. Bio-camouflage of anatase nanoparticles explored by in situ high-resolution electron microscopy. Nanoscale 2017, 9, 10684–10693. 10.1039/c7nr02239e. [DOI] [PubMed] [Google Scholar]
- Liang L.; Krieg P.; Rupp F.; Kimmerle-Müller E.; Spintzyk S.; Richter M.; Richter G.; Killinger A.; Geis-Gerstorfer J.; Scheideler L. Osteoblast Response to Different UVA-Activated Anatase Implant Coatings. Adv. Mater. Interfaces 2019, 6, 1801720. 10.1002/admi.201801720. [DOI] [Google Scholar]
- Cizek J.; Kovarik O.; Siska F.; Bensch J.; Cupera J.; Matejkova M.; Siegl J.; Chraska T.; Khor K. A. Increasing Fatigue Endurance of Hydroxyapatite and Rutile Plasma Sprayed Biocomponents by Controlling Deposition In-Flight Properties. ACS Biomater. Sci. Eng. 2019, 5, 1703–1714. 10.1021/acsbiomaterials.8b01545. [DOI] [PubMed] [Google Scholar]
- Tsyganov I.; Maitz M. F.; Wieser E. Blood Compatibility of Titanium-Based Coatings Prepared by Metal Plasma Immersion Ion Implantation and Deposition. Appl. Surf. Sci. 2004, 235, 156–163. 10.1016/j.apsusc.2004.05.134. [DOI] [Google Scholar]
- Verma A.; Uzun O.; Hu Y.; Hu Y.; Han H.-S.; Watson N.; Chen S.; Irvine D. J.; Stellacci F. Surface-Structure-Regulated Cell-Membrane Penetration by Monolayer-Protected Nanoparticles. Nat. Mater. 2008, 7, 588–595. 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson H. L.; Gustafsson J.; Cronholm P.; Möller L. Size-Dependent Toxicity of Metal Oxide Particles-A Comparison between Nano- and Micrometer Size. Toxicol. Lett. 2009, 188, 112–118. 10.1016/j.toxlet.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Trewyn B. G.; Nieweg J. A.; Zhao Y.; Lin V. S.-Y. Biocompatible Mesoporous Silica Nanoparticles with Different Morphologies for Animal Cell Membrane Penetration. Chem. Eng. J. 2008, 137, 23–29. 10.1016/j.cej.2007.09.045. [DOI] [Google Scholar]
- Wang L.; Zhou B.; Liu Z.; Dong L.; Cheng K.; Weng W. Surface Hydroxylation Regulates Cellular Osteogeneses on TiO2 and Ta2O5 Nanorod Films. Colloids Surf., B 2018, 167, 213–219. 10.1016/j.colsurfb.2018.04.012. [DOI] [PubMed] [Google Scholar]
- Bernier M.-C.; El Kirat K.; Besse M.; Morandat S.; Vayssade M. Preosteoblasts and Fibroblasts Respond Differently to Anatase Titanium Dioxide Nanoparticles: A Cytotoxicity and Inflammation Study. Colloids Surf., B 2012, 90, 68–74. 10.1016/j.colsurfb.2011.09.044. [DOI] [PubMed] [Google Scholar]
- Weir A.; Westerhoff P.; Fabricius L.; Hristovski K.; Von Goetz N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch I.; Salvati A.; Dawson K. A. Protein-Nanoparticle Interactions: What Does the Cell See?. Nat. Nanotechnol. 2009, 4, 546–547. 10.1038/nnano.2009.248. [DOI] [PubMed] [Google Scholar]
- Chen D.; Cao L.; Huang F.; Imperia P.; Cheng Y.-B.; Caruso R. A. Synthesis of Monodisperse Mesoporous Titania Beads with Controllable Diameter, High Surface Areas, and Variable Pore Diameters (14–23 nm). J. Am. Chem. Soc. 2010, 132, 4438–4444. 10.1021/ja100040p. [DOI] [PubMed] [Google Scholar]
- Tubío C. R.; Guitián F.; Salgueiro J. R.; Gil A. Anatase and Rutile TiO2 Monodisperse Microspheres by Rapid Thermal Annealing: A Method to Avoid Sintering at High Temperatures. Mater. Lett. 2015, 141, 203–206. 10.1016/j.matlet.2014.11.063. [DOI] [Google Scholar]
- Kokubo T.; Takadama H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity?. Biomaterials 2006, 27, 2907–2915. 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Simão A. M. S.; Beloti M. M.; Rosa A. L.; de Oliveira P. T.; Granjeiro J. M.; Pizauro J. M.; Ciancaglini P. Culture of Osteogenic Cells from Human Alveolar Bone: A Useful Source of Alkaline Phosphatase. Cell Biol. Int. 2007, 31, 1405–1413. 10.1016/j.cellbi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Gregory C. A.; Grady Gunn W.; Peister A.; Prockop D. J. An Alizarin Red-Based Assay of Mineralization by Adherent Cells in Culture: Comparison with Cetylpyridinium Chloride Extraction. Anal. Biochem. 2004, 329, 77–84. 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Zhao W.; Michalik D.; Ferguson S.; Hofstetter W.; Lemaître J.; von Rechenberg B.; Bowen P. Rapid Evaluation of Bioactive Ti-Based Surfaces Using an in Vitro Titration Method. Nat. Commun. 2019, 10, 2062. 10.1038/s41467-019-09673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubo T.; Miyaji F.; Kim H.-M.; Nakamura T. Spontaneous Formation of Bonelike Apatite Layer on Chemically Treated Titanium Metals. J. Am. Ceram. Soc. 1996, 79, 1127–1129. 10.1111/j.1151-2916.1996.tb08561.x. [DOI] [Google Scholar]
- Wick G.; Backovic A.; Rabensteiner E.; Plank N.; Schwentner C.; Sgonc R. The Immunology of Fibrosis: Innate and Adaptive Responses. Trends Immunol. 2010, 31, 110–119. 10.1016/j.it.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takadama H.; Kim H.-M.; Kokubo T.; Nakamura T. TEM-EDX Study of Mechanism of Bonelike Apatite Formation on Bioactive Titanium Metal in Simulated Body Fluid. J. Biomed. Mater. Res. 2001, 57, 441–448. . [DOI] [PubMed] [Google Scholar]
- Kim H.-M.; Himeno T.; Kawashita M.; Lee J.-H.; Kokubo T.; Nakamura T. Surface Potential Change in Bioactive Titanium Metal during the Process of Apatite Formation in Simulated Body Fluid. J. Biomed. Mater. Res., Part A 2003, 67, 1305–1309. 10.1002/jbm.a.20039. [DOI] [PubMed] [Google Scholar]
- Uchida M.; Kim H.-M.; Kokubo T.; Fujibayashi S.; Nakamura T. Structural dependence of apatite formation on titania gels in a simulated body fluid. J. Biomed. Mater. Res., Part A 2002, 64, 164–170. 10.1002/jbm.a.10414. [DOI] [PubMed] [Google Scholar]
- Yang B.; Uchida M.; Kim H. M.; Zhang X.; Kokubo T. Preparation of Bioactive Titanium Metal via Anodic Oxidation Treatment. Biomaterials 2004, 25, 1003–1010. 10.1016/s0142-9612(03)00626-4. [DOI] [PubMed] [Google Scholar]
- Wu J.-M.; Hayakawa S.; Tsuru K.; Osaka A. Low-Temperature Preparation of Anatase and Rutile Layers on Titanium Substrates and Their Ability to Induce in Vitro Apatite Deposition. J. Am. Ceram. Soc. 2004, 87, 1635–1642. 10.1111/j.1551-2916.2004.01635.x. [DOI] [Google Scholar]
- Abuna R. P. F.; Oliveira F. S.; Lopes H. B.; Freitas G. P.; Fernandes R. R.; Rosa A. L.; Beloti M. M. The Wnt/β-catenin signaling pathway is regulated by titanium with nanotopography to induce osteoblast differentiation. Colloids Surf., B 2019, 184, 110513. 10.1016/j.colsurfb.2019.110513. [DOI] [PubMed] [Google Scholar]
- Hong D.; Chen H.-X.; Yu H.-Q.; Liang Y.; Wang C.; Lian Q.-Q.; Deng H.-T.; Ge R.-S. Morphological and Proteomic Analysis of Early Stage of Osteoblast Differentiation in Osteoblastic Progenitor Cells. Exp. Cell Res. 2010, 316, 2291–2300. 10.1016/j.yexcr.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinger O.; Anselme K.; Denzer A.; Habersetzer P.; Wieland M.; Jeanfils J.; Hardouin P.; Landolt D. Time-Dependent Morphology and Adhesion of Osteoblastic Cells on Titanium Model Surfaces Featuring Scale-Resolved Topography. Biomaterials 2004, 25, 2695–2711. 10.1016/j.biomaterials.2003.09.111. [DOI] [PubMed] [Google Scholar]
- Uggeri J.; Guizzardi S.; Scandroglio R.; Gatti R. Adhesion of Human Osteoblasts to Titanium: A Morpho-Functional Analysis with Confocal Microscopy. Micron 2010, 41, 210–219. 10.1016/j.micron.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Suphasiriroj W.; Yotnuengnit P.; Surarit R.; Pichyangkura R. The Fundamental Parameters of Chitosan in Polymer Scaffolds Affecting Osteoblasts (MC3T3-E1). J. Mater. Sci.: Mater. Med. 2009, 20, 309–320. 10.1007/s10856-008-3575-2. [DOI] [PubMed] [Google Scholar]
- Andersson P. O.; Lejon C.; Ekstrand-Hammarström B.; Akfur C.; Ahlinder L.; Bucht A.; Österlund L. Polymorph- and Size-Dependent Uptake and Toxicity of TiO2 Nanoparticles in Living Lung Epithelial Cells. Small 2011, 7, 514–523. 10.1002/smll.201001832. [DOI] [PubMed] [Google Scholar]
- Bottini M.; Mebarek S.; Anderson K. L.; Strzelecka-Kiliszek A.; Bozycki L.; Simão A. M. S.; Bolean M.; Ciancaglini P.; Pikula J. B.; Pikula S.; Magne D.; Volkmann N.; Hanein D.; Millán J. L.; Buchet R. Matrix Vesicles from Chondrocytes and Osteoblasts: Their Biogenesis, Properties, Functions and Biomimetic Models. Biochim. Biophys. Acta, Gen. Subj. 2018, 1862, 532–546. 10.1016/j.bbagen.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z.; Camalier C. E.; Nagashima K.; Chan K. C.; Lucas D. A.; Cruz M. J. D. E. L. A.; Gignac M.; Lockett S.; Issaq H. J.; Veenstra T. D.; et al. Analysis of the Extracellular Matrix Vesicle Proteome in Mineralizing Osteoblasts. J. Cell. Physiol. 2007, 210, 325–335. 10.1002/jcp.20826. [DOI] [PubMed] [Google Scholar]
- Stoehr L. C.; Gonzalez E.; Stampfl A.; Casals E.; Duschl A.; Puntes V.; Oostingh G. J. Shape Matters: Effects of Silver Nanospheres and Wires on Human Alveolar Epithelial Cells. Part. Fibre Toxicol. 2011, 8, 36. 10.1186/1743-8977-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallhov H.; Gabrielsson S.; Strømme M.; Scheynius A.; Garcia-Bennett A. E. Mesoporous Silica Particles Induce Size Dependent Effects on Human Dendritic Cells. Nano Lett. 2007, 7, 3576–3582. 10.1021/nl0714785. [DOI] [PubMed] [Google Scholar]
- Hsiao I.-L.; Huang Y.-J. Effects of Various Physicochemical Characteristics on the Toxicities of ZnO and TiO2 Nanoparticles toward Human Lung Epithelial Cells. Sci. Total Environ. 2011, 409, 1219–1228. 10.1016/j.scitotenv.2010.12.033. [DOI] [PubMed] [Google Scholar]
- Liu N.; Li K.; Li X.; Chang Y.; Feng Y.; Sun X.; Cheng Y.; Wu Z.; Zhang H. Crystallographic Facet-Induced Toxicological Responses by Faceted Titanium Dioxide Nanocrystals. ACS Nano 2016, 10, 6062–6073. 10.1021/acsnano.6b01657. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Yu W.; Jiang X.; Lv K.; Sun S.; Zhang F. Analysis of the Cytotoxicity of Differentially Sized Titanium Dioxide Nanoparticles in Murine MC3T3-E1 Preosteoblasts. J. Mater. Sci.: Mater. Med. 2011, 22, 1933–1945. 10.1007/s10856-011-4375-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Contreras A.; Guadarrama Bello D.; Nanci A. Surface nanoporosity has a greater influence on osteogenic and bacterial cell adhesion than crystallinity and wettability. Appl. Surf. Sci. 2018, 445, 255–261. 10.1016/j.apsusc.2018.03.150. [DOI] [Google Scholar]
- Emer M.; Cardoso M. B. Biomolecular Corona Formation: Nature and Bactericidal Impact on Surface-Modified Silica Nanoparticles. J. Mater. Chem. B 2017, 5, 8052–8059. 10.1039/c7tb01744h. [DOI] [PubMed] [Google Scholar]
- Sacchetti C.; Motamedchaboki K.; Magrini A.; Palmieri G.; Mattei M.; Bernardini S.; Rosato N.; Bottini N.; Bottini M. Surface Polyethylene Glycol Conformation Influences the Protein Corona of Polyethylene Glycol-Modified Single-Walled Carbon Nanotubes: Potential Implications on Biological Performance. ACS Nano 2013, 7, 1974–1989. 10.1021/nn400409h. [DOI] [PubMed] [Google Scholar]
- Deligianni D.; Katsala N.; Ladas S.; Sotiropoulou D.; Amedee J.; Missirlis Y. F. Effect of Surface Roughness of the Titanium Alloy Ti-6Al-4V on Human Bone Marrow Cell Response and on Protein Adsorption. Biomaterials 2001, 22, 1241–1251. 10.1016/s0142-9612(00)00274-x. [DOI] [PubMed] [Google Scholar]
- Tedja R.; Lim M.; Amal R.; Marquis C. Effects of Serum Adsorption on Cellular Uptake Profile and Consequent Impact of Titanium Dioxide Nanoparticles on Human Lung Cell Lines. ACS Nano 2012, 6, 4083–4093. 10.1021/nn3004845. [DOI] [PubMed] [Google Scholar]
- Bhattacharya K.; Mukherjee S. P.; Gallud A.; Burkert S. C.; Bistarelli S.; Bellucci S.; Bottini M.; Star A.; Fadeel B. Biological Interactions of Carbon-Based Nanomaterials: From Coronation to Degradation. Nanomed.: Nanotechnol. Biol. Med. 2016, 12, 333–351. 10.1016/j.nano.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monopoli M. P.; Walczyk D.; Campbell A.; Elia G.; Lynch I.; Baldelli Bombelli F.; Dawson K. A. Physical-Chemical Aspects of Protein Corona: Relevance to in Vitro and in Vivo Biological Impacts of Nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. 10.1021/ja107583h. [DOI] [PubMed] [Google Scholar]
- Casals E.; Pfaller T.; Duschl A.; Oostingh G. J.; Puntes V. Time Evolution of the Nanoparticle Protein Corona. ACS Nano 2010, 4, 3623–3632. 10.1021/nn901372t. [DOI] [PubMed] [Google Scholar]
- Mahl D.; Greulich C.; Meyer-Zaika W.; Köller M.; Epple M. Gold Nanoparticles: Dispersibility in Biological Media and Cell-Biological Effect. J. Mater. Chem. 2010, 20, 6176–6181. 10.1039/c0jm01071e. [DOI] [Google Scholar]
- Ribeiro A. R.; Gemini-Piperni S.; Travassos R.; Lemgruber L.; Silva R. C.; Rossi A. L.; Farina M.; Anselme K.; Shokuhfar T.; Shahbazian-Yassar R.; et al. Trojan-Like Internalization of Anatase Titanium Dioxide Nanoparticles by Human Osteoblast Cells. Sci. Rep. 2016, 6, 23615. 10.1038/srep23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadeel B.; Garcia-Bennett A. E. Better Safe than Sorry: Understanding the Toxicological Properties of Inorganic Nanoparticles Manufactured for Biomedical Applications. Adv. Drug Deliv. Rev. 2010, 62, 362–374. 10.1016/j.addr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Keselowsky B. G.; Collard D. M.; García A. J. Surface Chemistry Modulates Fibronectin Conformation and Directs Integrin Binding and Specificity to Control Cell Adhesion. J. Biomed. Mater. Res., Part A 2003, 66, 247–259. 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- Chen L.; Simpson J. D.; Fuchs A. V.; Rolfe B. E.; Thurecht K. J. Effects of Surface Charge of Hyperbranched Polymers on Cytotoxicity, Dynamic Cellular Uptake and Localization, Hemotoxicity, and Pharmacokinetics in Mice. Mol. Pharmaceutics 2017, 14, 4485–4497. 10.1021/acs.molpharmaceut.7b00611. [DOI] [PubMed] [Google Scholar]
- Shemetov A. A.; Nabiev I.; Sukhanova A. Molecular Interaction of Proteins and Peptides with Nanoparticles. ACS Nano 2012, 6, 4585–4602. 10.1021/nn300415x. [DOI] [PubMed] [Google Scholar]
- Lundqvist M.; Stigler J.; Elia G.; Lynch I.; Cedervall T.; Dawson K. A. Nanoparticle Size and Surface Properties Determine the Protein Corona with Possible Implications for Biological Impacts. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 14265–14270. 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkilany A. M.; Nagaria P. K.; Hexel C. R.; Shaw T. J.; Murphy C. J.; Wyatt M. D. Cellular Uptake and Cytotoxicity of Gold Nanorods: Molecular Origin of Cytotoxicity and Surface Effects. Small 2009, 5, 701–708. 10.1002/smll.200801546. [DOI] [PubMed] [Google Scholar]
- Lv L.; Li K.; Xie Y.; Cao Y.; Zheng X. Enhanced osteogenic activity of anatase TiO2 film: Surface hydroxyl groups induce conformational changes in fibronectin. Mater. Sci. Eng., C 2017, 78, 96–104. 10.1016/j.msec.2017.04.056. [DOI] [PubMed] [Google Scholar]
- Gagner J. E.; Qian X.; Lopez M. M.; Dordick J. S.; Siegel R. W. Effect of Gold Nanoparticle Structure on the Conformation and Function of Adsorbed Proteins. Biomaterials 2012, 33, 8503–8516. 10.1016/j.biomaterials.2012.07.009. [DOI] [PubMed] [Google Scholar]