Abstract
Introduction
Individuals with Alzheimer's disease (AD) broadly exhibit lower cardiorespiratory fitness (CRF) compared to cognitively healthy older adults. Other factors, such as increasing age and female sex, are also known to track with lower CRF levels. However, it is unclear how these factors together with AD diagnosis and genetic risk (apolipoprotein e4 ; APOE4) collectively affect CRF.
Methods
Our primary objective was to characterize the collective relationship of age, sex, APOE4 carrier status , and cognitive status (nondemented or AD) with two commonly reported CRF outcomes, VO2 max and oxygen uptake efficiency slope (OUES). To interrogate the unique and combined effect of age, sex, APOE4, and cognitive status on CRF, we pooled multiple datasets and tested several statistical models allowing all possible interactions.
Results
AD diagnosis was consistently associated with lower maximal CRF, which declined with increasing age. APOE4 was also associated with lower maximal CRF (VO2max), but only in male subjects. Submaximal CRF (OUES) was lower in APOE4 carriers of both sexes, although this difference converged in male subjects with advancing age.
Discussion
This multi‐cohort analysis (n = 304) suggests that APOE4 carrier status and sex are important considerations for studies that evaluate maximal and submaximal CRF.
Keywords: aging, Alzheimer's disease, apolipoprotein e4, cardiorespiratory fitness, oxygen uptake efficiency slope
1. INTRODUCTION
Low cardiorespiratory fitness (CRF) and physical inactivity have been linked to Alzheimer's disease (AD), 1 , 2 , 3 , 4 and longitudinal studies have shown a relationship between self‐reported physical activity and exercise (planned repetitive physical activity for the purpose of health), and sustained cognitive function. 5 , 6 , 7 , 8 , 9 , 10 Higher lifetime physical activity, which has been shown to contribute to greater CRF levels in later life 11 is associated with reduced AD risk, 12 , 13 and in individuals with elevated AD risk, higher CRF has been associated with greater brain volume and better cognitive performance. 14 In two separate clinical trials, we have shown that aerobic exercise interventions designed to increase CRF improve cognitive function in nondemented older adults in a dose‐dependent manner, and that exercise‐induced improvement in CRF is positively associated with both memory and hippocampal volume in AD. 15 , 16
Despite these positive findings, it is clear that some individuals experience more brain benefit from physical activity and exercise than others, and this response is particularly variable in participants with AD. 16 , 17 Age, sex, and AD status have all been individually linked to CRF, but it is unclear how these factors, collectively with AD genetic risk status, such as apolipoprotein E4 (APOE4) carrier status, relate to CRF responses in humans. We sought to further interrogate the independent and combined effects of these key factors to determine their relationships with two commonly used indices of CRF. The first measure, VO2 max, is considered the “gold standard” measure of CRF in exercise trials but requires expertise to perform and is not feasible for all research participants. A less intensive measure that does not require achieving maximal effort, oxygen uptake efficiency slope (OUES), is more feasible and likely safer for some participants. 18 , 19 Given the links between CRF and AD risk, as well as the heterogeneous nature of study subjects and observed CRF responses, we aimed to characterize the collective impacts of age, sex, APOE4 carrier status, and cognitive status on commonly reported CRF outcomes.
2. METHODS
2.1. Participants
Participants were recruited as part of intervention and observational studies at the University of Kansas Alzheimer's Disease Center (KU) and the Wisconsin Alzheimer's Disease Research Center (UW). We have previously reported results from these investigations. 15 , 16 , 20 , 21 , 22 Briefly, both sites are part of the U.S. network of Alzheimer's Disease Centers of Excellence that support research into brain aging and dementia. All work was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving human subjects. All human subjects provided informed consent.
RESEARCH IN CONTEXT
Systematic review: A traditional literature review (PubMed) was performed to identify relevant studies. It has been shown that Alzheimer's disease (AD) subjects have lower cardiorespiratory fitness (CRF) levels compared to cognitively healthy individuals. However, CRF is also affected by a variety of other factors, including age and sex, and there is limited information regarding the effect of the primary AD risk gene, apolipoprotein E epsilon 4 (APOE4), on CRF.
Interpretation: Here, we interrogated the independent and combined contributions of age, sex, cognitive diagnosis, and APOE4 carrier status to examine the effects of these factors on both maximal (VO2 max) and submaximal/functional fitness (oxygen uptake efficiency slope). APOE4 was associated with lower maximal fitness only in male subjects, but was associated with lower functional fitness at younger ages regardless of sex.
Future directions: This article suggests that APOE4 genotype and sex should be a consideration factor for ongoing clinical trials examining CRF in aging and AD.
The KU sample was drawn from the most recent individual graded exercise testing (GXT) from both intervention (pre‐intervention timepoint only) and observational studies (ClinicalTrials.gov: NCT01129115, NCT02000583, NCT01128361, NCT00267124). The UW sample was drawn from participants enrolled in sub‐studies of the Wisconsin Registry for Alzheimer's Prevention study. 23 , 24 All individuals were evaluated using the Clinical Dementia Rating (CDR) and determined to have either nondemented (ND) or cognitive impairment due early‐stage AD (CDR 0.5 or 1; AD). We included ND subjects 50 years and older and AD subjects of any age. We pooled GXT results from the baseline assessments for past exercise studies and clinical data from 510 individuals between our two sites. We then excluded 206 individuals who did not achieve a respiratory exchange ratio (RER) of ≥1.10 during the GXT, a well‐accepted threshold for an excellent effort during an exercise test. 25 The remaining 304 individuals (219 ND, 85 AD) were included in our final analyses. For these individuals, we also assessed additional criteria that could impact our results, including the rating of perceived exertion (RPE) and use of beta‐blockers. These data, along with clinical data, are included in Table 1.
TABLE 1.
Demographics and graded exercise testing performance
| Female | Male | |||||||
|---|---|---|---|---|---|---|---|---|
| AD | ND | AD | ND | |||||
| E4 Carrier | E4 Noncarrier | E4 Carrier | E4 Noncarrier | E4 Carrier | E4 Noncarrier | E4 Carrier | E4 Noncarrier | |
| Number, n | 30 | 9 | 53 | 82 | 22 | 24 | 26 | 58 |
| Age (years) | 73.1 (7.6) | 73.8 (5.4) | 66.6 (7.0) | 70.4 (6.6) | 72.6 (7.8) | 72.3 (7.8) | 70.1 (8.4) | 72.5 (7.6) |
| RER | 1.14 (0.06) | 1.15 (0.05) | 1.16 (0.05) | 1.15 (0.06) | 1.16 (0.07) | 1.16 (0.05) | 1.16 (0.05) | 1.17 (0.8) |
| RPE | 17.8 (1.7) | 17.7 (1.5) | 17.5 (1.7) | 17.8 (1.3) | 17.9 (1.6) | 17.4 (1.8) | 17.9 (1.2) | 17.4 (1.9) |
| Beta‐blocker (#,%) | 3 (10%) | 1 (11.1%) | 3 (5.7%) | 2 (2.4%) | 2 (9.1%) | 1 (4.2%) | 1 (3.8%) | 3 (5.2%) |
| VO2max (mL/kg/min) | 19.3 (3.0) | 18.9 (3.1) | 22.5 (4.5) | 20.9 (4.5) | 22.0 (4.0) | 24.2 (5.1) | 25.8 (5.8) | 26.1 (5.2) |
| OUES | 1348 (209) | 1543 (219) | 1715 (394) | 1608 (399) | 1987 (529) | 2182 (641) | 2436 (673) | 2265 (555) |
Notes: Characteristics of participants who met RER criteria for inclusion in our analysis (n = 304) broken down by categorical variables (sex, cognitive diagnosis, and E4 carrier status). Data are shown as means and SD.
Abbreviations: AD, Alzheimer's disease; ND, nondemented; E4, apolipoprotein e4; RER, respiratory exchange ratio; RPE, rating of perceived exertion; OUES, oxygen uptake efficiency slope.
2.2. Assessment and measures
CRF was indexed as the maximal oxygen uptake during the GXT normalized to whole body mass (mL*kg−1*min−1). Treadmill speed and incline changes were preset and controlled by the metabolic analysis software. Expired gases were captured using a nose clip or mask, mouthpiece, and 2‐way nonrebreathing valve. Participants were oriented to the Borg Rating of RPE 6 to 20 scale before beginning the GXT. GXT outcome measures were obtained using the Balke protocol at UW and the modified Bruce protocol at KU. Both sites used American College of Sports Medicine recommendations for absolute and relative indications for terminating tests. Details can be found in the parent datasets. 15 , 16 , 20 , 22 OUES, a valid estimate of cardiorespiratory efficiency, was calculated by regressing oxygen uptake on the log transformed total ventilation at each 15‐second sampling period of the entire GXT. 26
2.3. Statistical analyses
Participant characteristics were explored with standard descriptive statistics. We then performed a backward elimination for ordinary least squares regression modeling to predict VO2 max and OUES. We included cognitive status (ND/AD), APOE4 status (E4 carrier/noncarrier), and sex (male/female) as dichotomous variables, as well as age in years as a continuous measure. We began our backward elimination with an inclusive model allowing for all possible interactions (ie, up to the 4‐way interaction among diagnosis, APOE4 carrier status, sex, and age). Given the known strong effect of sex on CRF outcomes, and its significance in the full model, we then performed subgroup analyses separating males and females to explore the relationship of the remaining variables (age, APOE4, and cognitive status) on CRF. We iteratively tested these reduced models to identify the most parsimonious predictors of VO2 max and OUES.
We used a threshold of P = .2 for backward elimination steps. We then assessed for multicollinearity. When condition indices exceeded 100, we reduced backward elimination to a lower P value (P –.15) and reassessed condition indices. 27 Adjusted R‐square values were estimated from these models to indicate the level of variation in these measures described. Last, to assess model assumptions, we conducted residual analyses, including assessments for normality for residuals using histograms and quantile‐quantile plots, and for homogeneity of variance evaluated residual by predicted value plots. We considered the following complete and reduced models for VO2 max and OUES outcomes, below.
Full model for VO2 max:
Reduced model for VO2 max (after backward selection; ):
Full model for OUES:
Reduced model for OUES (after backward selection; ):
3. RESULTS
3.1. Cohort characteristics
As expected, the two cohorts differed in age (P < .001). Because the UW cohort was 9.7 years younger on average and were not diagnosed with cognitive impairment, the observed higher VO2 max and OUES values were expected (P < .01). The UW cohort also had a slightly higher proportion of females (P = .04).
Across both cohorts (Table 1), females were younger (P = .015) and had lower CRF (P < .001). Females also had a higher rate of APOE4 carriage and slightly lower RER at VO2 max, though these did not reach significance (P > .06). Individuals diagnosed with cognitive impairment were older, more likely to carry the E4 allele, and have lower VO2max (P < .05) and marginally lower OUES (P = .07). E4 carriers were slightly older (P = .05), but did not differ on RER, VO2 max, or OUES (P > .2).
3.2. Regression analyses
The full regression model indicated that female sex, APOE4 carriage, and diagnosis all contributed to lower CRF. Because large effects of the categorical variable sex on both VO2 max and OUES are well established, we elected to further explore the collective contribution of our other variables (age, cognitive status, and APOE4) on CRF on males and females separately. Table 2 contains regression model results for CRF outcomes for these analyses.
TABLE 2.
Regression model results for CRF outcomes
| Subgroups | Linear relationship (P value) | Slope (P value, estimate) | Intercept (P value, estimate) | |
|---|---|---|---|---|
| Male subjects | ||||
| VO2max |
(ND, E4) vs (ND, Non E4) |
.044 |
.044 −1.481 |
|
|
(AD, E4) vs (AD, Non E4) |
.044 |
.044 −1.481 |
||
| OUES |
(ND, E4) vs (ND, Non E4) |
.026 |
0.009 16.855 |
.012 −1133.793 |
|
(AD, E4) vs (AD, Non E4) |
<.001 |
0.009 16.855 |
.002 −1430.924 |
|
| Female subjects | ||||
| VO2 max |
(ND, E4) vs (ND, Non E4) |
.124 |
0.101 0.144 |
.044 −1.481 |
|
(AD, E4) vs (AD, Non E4) |
.124 |
0.295 −0.291 |
.044 −1.481 |
|
| OUES |
(ND, E4) vs (ND, Non E4) |
.026 |
0.009 16.855 |
.012 −1133.793 |
|
(AD, E4) vs (AD, Non E4) |
<.001 |
0.009 16.855 |
.002 −1430.924 |
|
Notes: Regression model values are given for cardiorespiratory fitness outcomes using threshold P = .015 for backward elimination.
Abbreviations: CRF, cardiorespiratory fitness; OUES, oxygen uptake efficiency slope.
3.3. Male subjects
Males who were APOE4 carriers showed lower VO2 max (P = .044; Figure 1A) both for comparisons within AD and within ND individuals. Diagnosis of AD was also associated with reliably lower VO2 max (P = .002) for both within E4 carriers and noncarriers (Table 2). Across all males, we observed the expected negative relationships between age and VO2 max. This decline with advancing age did not differ by carrier status.
FIGURE 1.
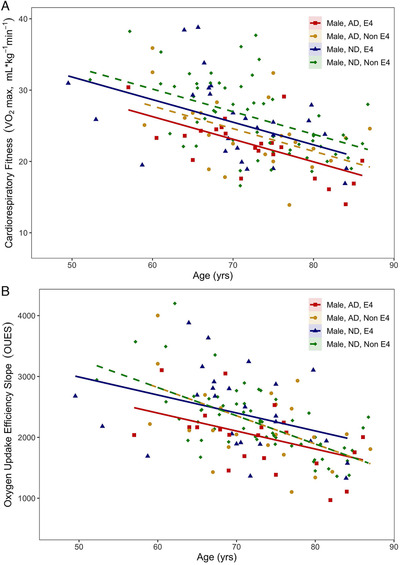
A, VO2 max and (B) oxygen uptake efficiency slope values for all male participants plotted by age and shown with the corrected regression line of best fit
Similarly, age was negatively associated with OUES across all males. OUES is also different between APOE4 genetic risk groups in both ND and AD participants. Regardless of diagnosis younger APOE4 carriers showed lower OUES (P < .03; Figure 1B) at an earlier age, with OUES values converging with advancing age (ie, noncarrier OUES dropping more rapidly).
3.4. Female subjects
Unlike male subjects, female APOE4 carriers did not exhibit differences in VO2 max compared to noncarriers (Figure 2A). At young ages differences in VO2 max were observed in AD females and marginally converged as VO2 max values declined with advancing age (Table 2). The decline in VO2 max with advancing age did not differ by APOE4 carrier status.
FIGURE 2.
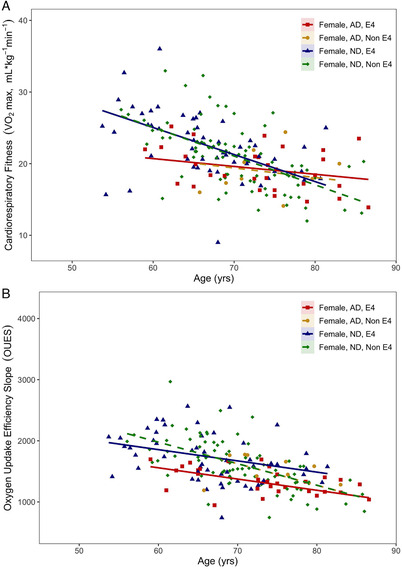
A, VO2 max and (B) oxygen uptake efficiency slope values for all female participants plotted by age and shown with the corrected regression line of best fit
Consistent with the findings in males, OUES was significantly different based on APOE4 carrier status in both ND (P = .026) and AD (P < .001) participants. Lower OUES was observed at younger ages in APOE4 carriers (Figure 2B).
4. DISCUSSION
We present here the first study to characterize how varying age, cognitive status, APOE genotype, and sex affect CRF. Given the heterogeneity of cohorts enrolled into ongoing clinical trials, it is important to understand these relationships. Our analysis revealed several interesting findings specifically regarding APOE4, the primary late onset AD risk gene. Regardless of diagnosis, positive APOE4 carrier status was associated with lower maximal fitness (VO2 max) in male subjects, but not in female subjects. However, APOE4 was associated with lower submaximal fitness (OUES) at younger ages, regardless of sex.
VO2 max is the gold standard for measuring CRF and is a marker of the body's maximal ability to transport and use oxygen. Thus, VO2 max reflects a combination of both cardiovascular function and energy demand and use. We show here that both ND and AD male APOE4 carriers had marginally lower VO2 max overall than APOE4 noncarriers. Interestingly, the rate of VO2 max decline with increasing age did not differ based upon APOE4 carrier status. No difference in VO2 max scores between risk groups was observed in female subjects of either diagnosis. This suggests that AD genetic risk status negatively affects an individual's baseline CRF value most strongly in males. In addition, APOE4 individuals (in both sexes) do not change differently over time. This provides important information for longitudinal trials assessing CRF.
In contrast, OUES is a function of both oxygen consumption and ventilation. Because OUES is affected by perfusion to both lungs and skeletal muscle it is said to reflect system efficiency. 26 Here, we show that at younger ages, APOE4 carriers have lower OUES values compared to APOE4 noncarriers, regardless of diagnosis group or sex. It is possible that these findings are affected by survival bias, as older APOE4 subjects do not have lower OUES. However, to our knowledge, no studies directly assess APOE4 genotype and OUES and additional work is needed to investigate this relationship.
Strengths of this study include our relatively large sample size, thorough diagnostic characterization, multiple cohorts, and robust characterization of CRF, using gold‐standard methods and accepted cut‐points for valid VO2 max values. Our values are in line with previously published norms, 28 with consideration of our clinical population. However, there are important study limitations that must be considered. First, we combined data collected at two sites to obtain a larger and more diverse sample set, but GXT protocols differed at the sites. Bruce and Balke GXT protocols have shown similar fitness values when compared directly, 29 but there is limited data comparing the modified Bruce and Balke protocols. In addition, even with our combined sites, additional weaknesses are the small number of female APOE4 noncarriers who met criteria for inclusion in the study, and the relatively low numbers of younger subjects due to the nature of the parent studies. Finally, we cannot rule out potential reverse causation due to functional impairments in AD. Our strict inclusion criteria of RER ≤1.10 likely minimizes protocol‐related and effort‐related differences, but may limit generalizability, thus we have included the demographic information for the full set of individuals considered for the study for comparison purposes (Table S1 in supporting information). Finally, there was limited quantification of other factors that could affect CRF, such as cardiovascular or metabolic comorbidities. Further studies should examine mechanisms for the effect of APOE4 differences on fitness.
In conclusion, we present here a relatively large, multi‐cohort study that characterizes the collective relationship of age, sex, cognitive status, and APOE4 carrier status on CRF. These findings suggest that APOE genotype and sex should be considered for ongoing clinical trials examining CRF in aging and AD.
CONFLICTS OF INTEREST
The authors have no competing interests to declare.
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors were supported by National Institute on Aging grants R00AG050490, R01AG050490, R21AG061548, R01AG052954, R01 AG062167, R21 AG051858, F31 AG062009, and R01 AG027161. This work was also supported by P30 AG035982 (KU ADC) and P30AG534255 (Wisconsin ADRC). Additional support was provided by funding from the Extendicare Foundation and the Alzheimer's Association (NIRGD‐305257). Portions of this research were also supported by the Veterans Administration including facilities and resources at the Geriatric Research Education and Clinical Center of the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin, USA.
Morris JK, Zhang G, Dougherty RJ, et al. Collective effects of age, sex, genotype, and cognitive status on fitness outcomes. Alzheimer's Dement. 2020;12:e12058 10.1002/dad2.12058
REFERENCES
- 1. Vidoni ED, Honea RA, Billinger SA, Swerdlow RH, Burns JM. Cardiorespiratory fitness is associated with atrophy in Alzheimer's and aging over 2 years. Neurobiol Aging. 2012;33(8):1624‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dougherty RJ, Schultz SA, Boots EA, et al. Relationships between cardiorespiratory fitness, hippocampal volume, and episodic memory in a population at risk for Alzheimer's disease. Brain Behav. 2017;7(3):e00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pentikainen H, Ngandu T, Liu Y, et al. Cardiorespiratory fitness and brain volumes in men and women in the FINGER study. Age Ageing. 2017;46(2):310‐313. [DOI] [PubMed] [Google Scholar]
- 4. Muller J, Chan K, Myers JN. Association between exercise capacity and late onset of dementia, Alzheimer disease, and cognitive impairment. Mayo Clin Proc. 2017;92(2):211‐217. [DOI] [PubMed] [Google Scholar]
- 5. Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498‐504. [DOI] [PubMed] [Google Scholar]
- 6. Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Internal Med. 2001;161(14):1703‐1708. [DOI] [PubMed] [Google Scholar]
- 7. Pignatti F, Rozzini R, Trabucchi M. Physical activity and cognitive decline in elderly persons. Arch Internal Med. 2002;162(3):361‐362. [DOI] [PubMed] [Google Scholar]
- 8. Albert MS, Jones K, Savage CR, et al. Predictors of cognitive change in older persons: macArthur studies of successful aging. Psychol Aging. 1995;10(4):578‐589. [DOI] [PubMed] [Google Scholar]
- 9. Weuve J, Kang JH, Manson JE, et al. Physical activity, including walking, and cognitive function in older women. JAMA 2004;292(12):1454‐1461. [DOI] [PubMed] [Google Scholar]
- 10. Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144(2):73‐81. [DOI] [PubMed] [Google Scholar]
- 11. Carrick‐Ranson G, Hastings JL, Bhella PS, et al. The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol (1985). 2014;116(7):736‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friedland RP, Fritsch T, Smyth KA, et al. Patients with Alzheimer's disease have reduced activities in midlife compared with healthy control‐group members. Proc Natl Acad Sci U S A. 2001;98(6):3440‐3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78(17):1323‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boots EA, Schultz SA, Oh JM, et al. Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle‐aged cohort at risk for Alzheimer's disease. Brain Imaging Behav. 2015;9(3):639‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vidoni ED, Johnson DK, Morris JK, et al. Dose‐response of aerobic exercise on cognition: a community‐based, pilot randomized controlled trial. PLoS One. 2015;10(7):e0131647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morris JK, Vidoni ED, Johnson DK, et al. Aerobic exercise for Alzheimer's disease: a randomized controlled pilot trial. PLoS One. 2017;12(2):e0170547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamb SE, Sheehan B, Atherton N, et al. Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ. 2018;361:e12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hollenberg M, Tager IB. Oxygen uptake efficiency slope: an index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. J. Am. Coll. Cardiol. 2000;36(1):194‐201. [DOI] [PubMed] [Google Scholar]
- 19. Dougherty RJ, Lindheimer JB, Stegner AJ, Riper SV, Okonkwo OC, Cook DB. An objective method to accurately measure cardiorespiratory fitness in older adults who cannot satisfy widely used oxygen consumption criteria. J Alzheimers Dis. 2018;61(2):601‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burns JM, Cronk BB, Anderson HS, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71(3):210‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schultz SA, Boots EA, Almeida RP, et al. Cardiorespiratory fitness attenuates the influence of amyloid on cognition. J Int Neuropsychol Soc. 2015;21(10):841‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dougherty RJ, Boots EA, Lindheimer JB, et al. Fitness, independent of physical activity is associated with cerebral blood flow in adults at risk for Alzheimer's disease. Brain Imaging Behav. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sager MA, Hermann B, La Rue A. Middle‐aged children of persons with Alzheimer's disease: aPOE genotypes and cognitive function in the Wisconsin registry for Alzheimer's prevention. J Geriatr Psychiatry Neurol. 2005;18(4):245‐249. [DOI] [PubMed] [Google Scholar]
- 24. Johnson SC, Koscik RL, Jonaitis EM, et al. The Wisconsin registry for Alzheimer's prevention: a review of findings and current directions. Alzheimers Dement (Amst). 2018;10:130‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hollenberg M, Ngo LH, Turner D, Tager IB. Treadmill exercise testing in an epidemiologic study of elderly subjects. J Gerontol A Biol Sci. 1998;53A(4):259‐267. [DOI] [PubMed] [Google Scholar]
- 26. Baba R, Nagashima M, Goto M, et al. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol. 1996;28(6):1567‐1572. [DOI] [PubMed] [Google Scholar]
- 27. Belsley DA, Kuh TD, Welsch RE. Regression Diagnostics. Hoboken, NJ, USA: John Wiley & Sons Inc.; 1980. [Google Scholar]
- 28. Kaminsky LA, Arena R, Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the fitness registry and the importance of exercise national database. Mayo Clin Proc. 2015;90(11):1515‐1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Froelicher VF, Brammell H, Davis G, Noguera I, , Steward A, Lancaster MC. A comparison of three maximal exercise protocols. J Appl Physiol. 1974;36(6):720‐725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


