Figure 1.
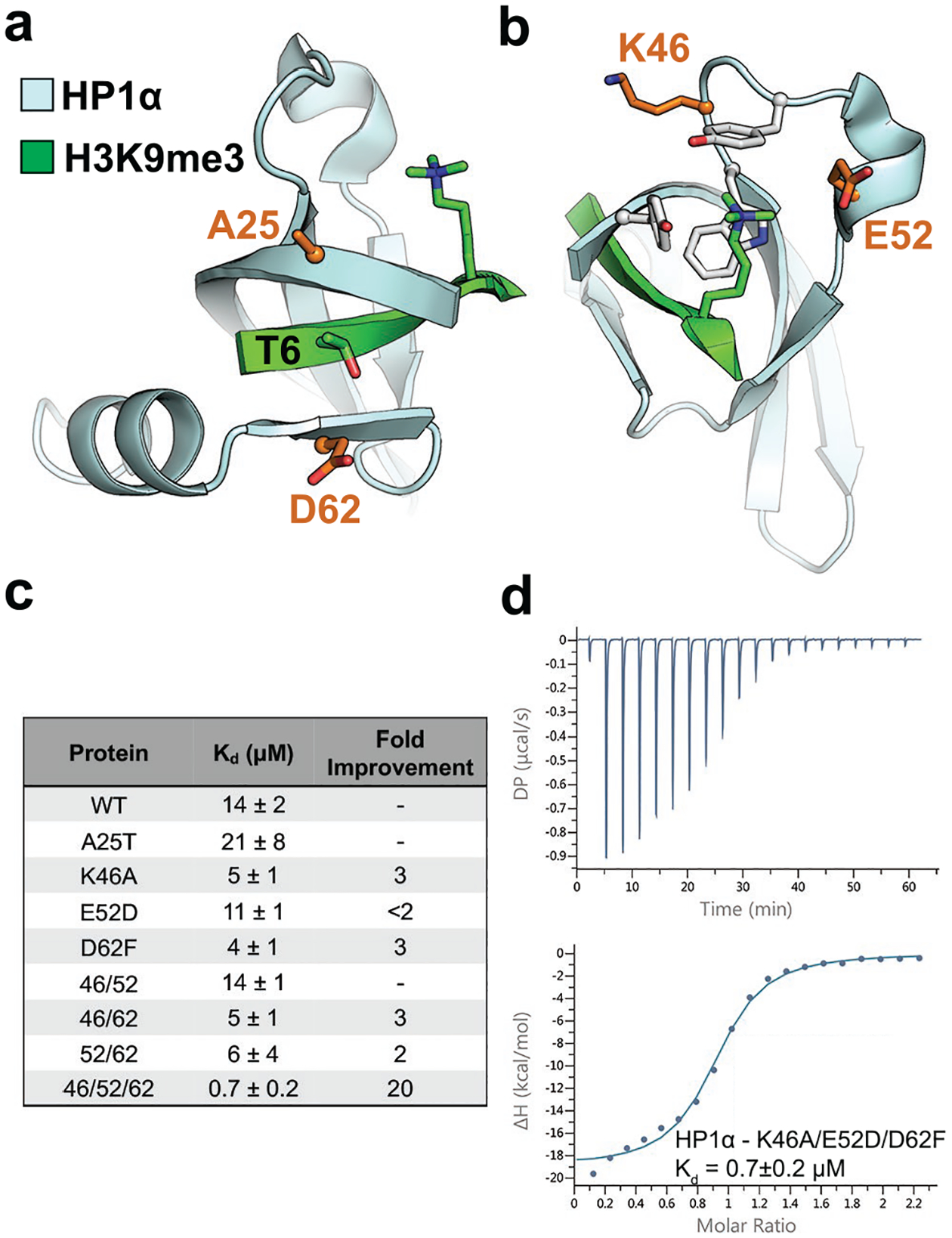
Rational mutation of the HP1α chromodomain increases binding affinity for H3K9me3. a) and b) HP1α chromodomain (cyan) recognizes H3K9me3 (green) through β-sheet formation with the histone tail and cation-π interactions with trimethylated lysine, and the mutated sites are highlighted in orange (PDB ID: 1KNE). c) Summary of results for ITC experiments for HP1α chromodomain variants showing the fold improvement in binding over wild-type. Data shown are representative of n ≥ 2 ITC binding experiments. d) ITC binding curve for the HP1α K46A/E52D/D62F engineered chromodomain.
