Figure 6. ARF GTPase activation by GDP/GTP exchange factors to regulate vesicle formation and unusual uncompetitive mechanism of action of BFA, which led to the concept of interfacial inhibition, where protein complexes are stabilized near interacting interfaces.
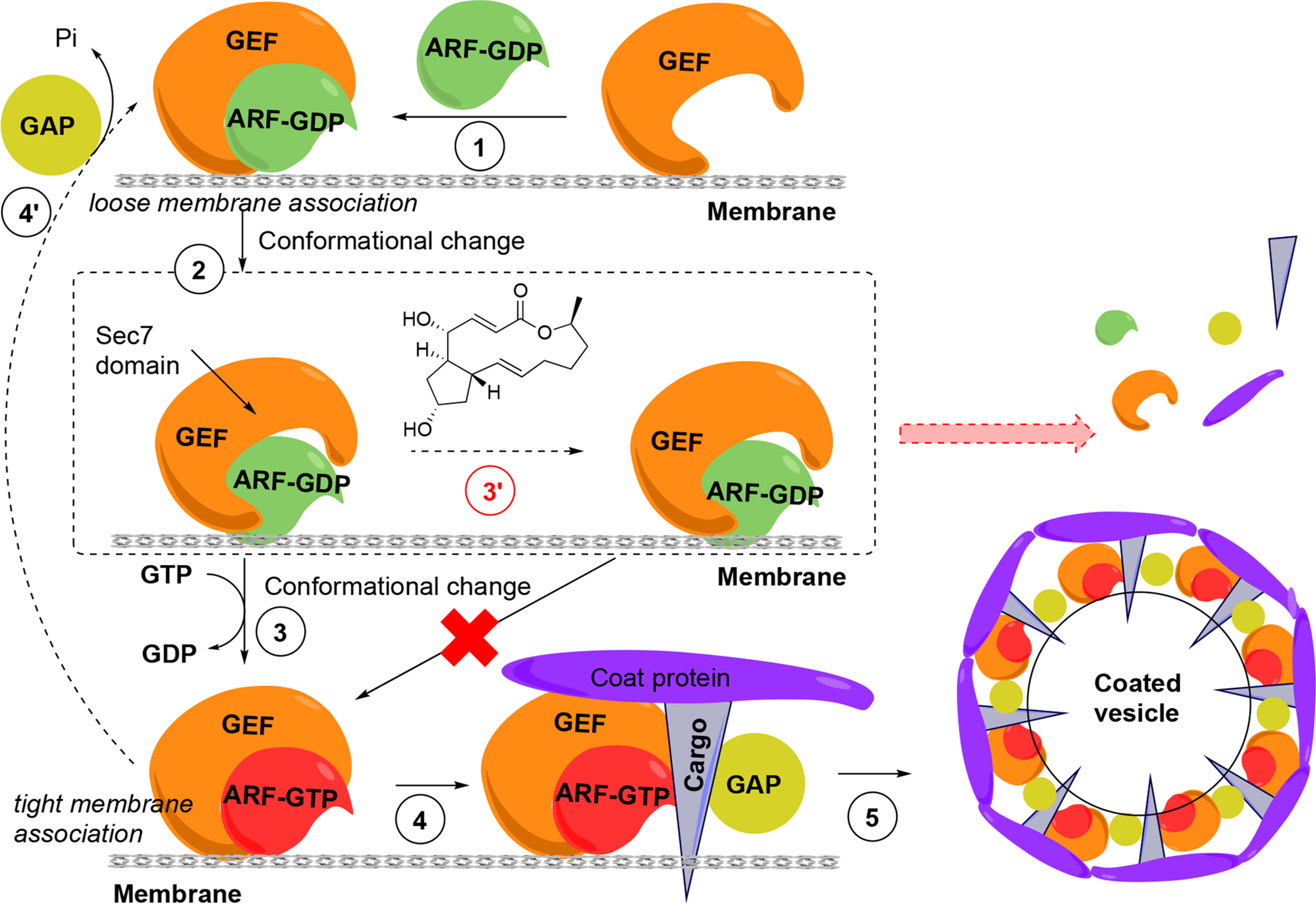
(1) GDP-bound ARF associates with GEF. (2) Conformational change of ARF is required to stimulate GDP dissociation. (3) GEF catalyzes the activation of ARF through GDP/GTP exchange and the complex now tightly associates with the donor membrane. (4) ARF-GTP recruits effectors, including coat components (coat proteins, cargo) from the cytosol, and also GTPase activating protein (GAP), which usually catalyzes the inactivation of ARF-GTP, closing the GDP-GTP cycle (4’). (5) GAP appears to be inactive when bound to the coat-containing complex, presumably driving coat polymerization, budding and vesicle formation. GAP becomes active in this conformational complex state, leading to GTP hydrolysis coat and GAP release and the uncoated vesicle is able to fuse with an acceptor membrane (not shown). (3’) In the presence of BFA the low-affinity complex is trapped through binding of BFA at the interface of ARF-GDP and the Sec7 domain of GEF. Binding of BFA to the Arf-GDP-Sec7 domain complex blocks GDP/GTP exchange on ARFs and consequently vesicle coat recruitment and vesicle formation. The ultimate result is the release of coat components into the cytosol and prevention of membrane trafficking.
