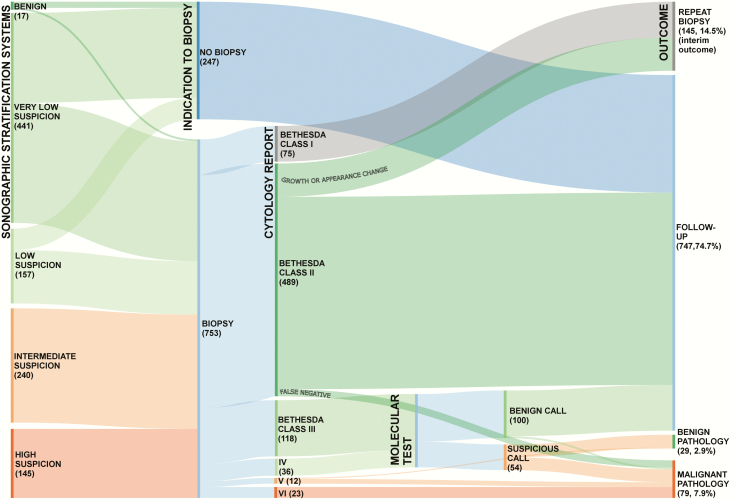
Figure 1.
Alluvial flow diagram showing simulated management and outcomes for 1000 newly discovered thyroid nodules. The distributions of ultrasound (US)-defined risk classes, US-defined fine-needle aspiration biopsy (FNAB) indications, and Bethesda cytology class were derived from published findings (21). US risk-stratification is that recommended by the American Thyroid Association (ATA) Guidelines. Nodules not classifiable with the ATA system are included in the intermediate-suspicion category. Nondiagnostic nodules with very-low-suspicion or low-suspicion US findings can be managed with US surveillance, but repeat FNAB is indicated for those with intermediate- or high-suspicion US findings (81): in this diagram, all are shown as undergoing repeat biopsy. Bethesda II nodules require repeat biopsy only if the US-based risk class increases during surveillance (frequency: ~15% over 5 years of follow-up) (82). The false-negative rate is less than 3% (e.g., sampling error; for high-suspicion nodules with Bethesda II cytology, repeat biopsy is suggested) (83). For illustration purposes, all indeterminate nodules are shown as undergoing molecular testing (regardless of other possible options). The hypothetical molecular testing approach depicted has a benign call rate of 65%, a positive predictive value of 50%, and a negative predictive value of 96% (72-74). For high-suspicion nodules classified as benign by molecular testing, repeat biopsy is indicated. All Bethesda V and VI nodules are referred for surgery. Expected malignancy rates are 80% and 99%, respectively.