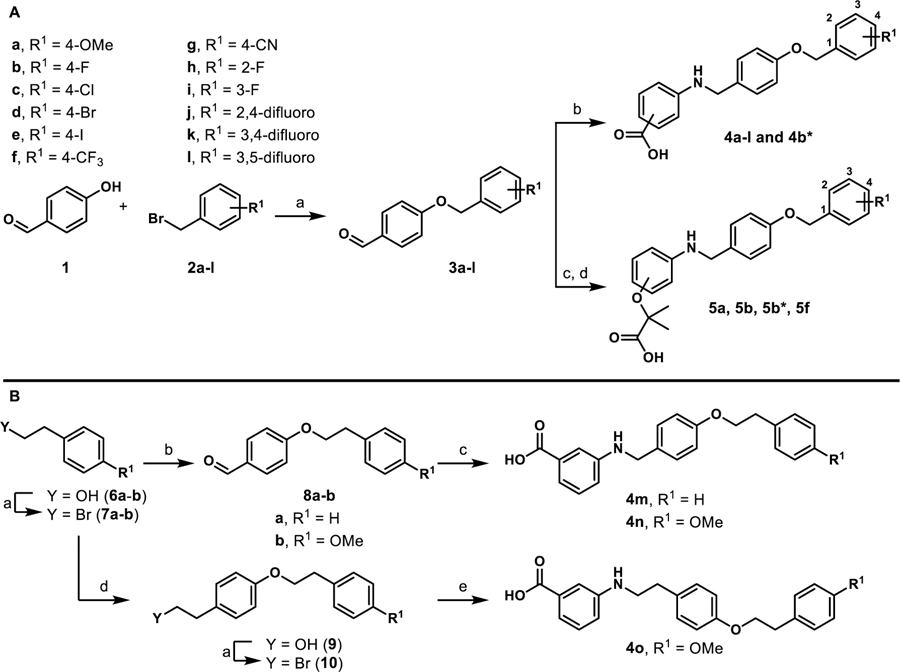
Scheme 1.
Synthesis of stage I analoguesa
aReagents and conditions: A) (a) K2CO3, DMF; (b) i) 3-aminobenzoic acid or 4-amino benzoic acid, toluene, 130 °C, 2–3 h; ii) NaBH(OAc)3, acetic acid, THF, 12 h, 32–97%; (c) i) ethyl 2-(3-aminophenoxy)-2-methylpropanoate or ethyl 2-(4-aminophenoxy)-2-methylpropanoate, toluene, 130 °C, 2–3 h; ii) NaBH(OAc)3, acetic acid, THF, 12 h; (d) LiOH·H2O, THF/CH3OH/H2O, 12 h, 14–60%. B) (a) PBr3, diethyl ether; (b) 4-hydroxybenzaldehyde, K2CO3, DMF, 40–65%; (c) 3-aminobenzoic acid, toluene, 130 °C, 2–3 h; ii) NaBH(OAc)3, acetic acid, THF, 12 h, 96%; (d) tyrosol, K2CO3, DMF, 26%; (e) 3-aminobenzoic acid, Et3N, DMF, 25%. All yields reported are unoptimized. Asterisks indicate the -COOH motif is attached para instead of meta.