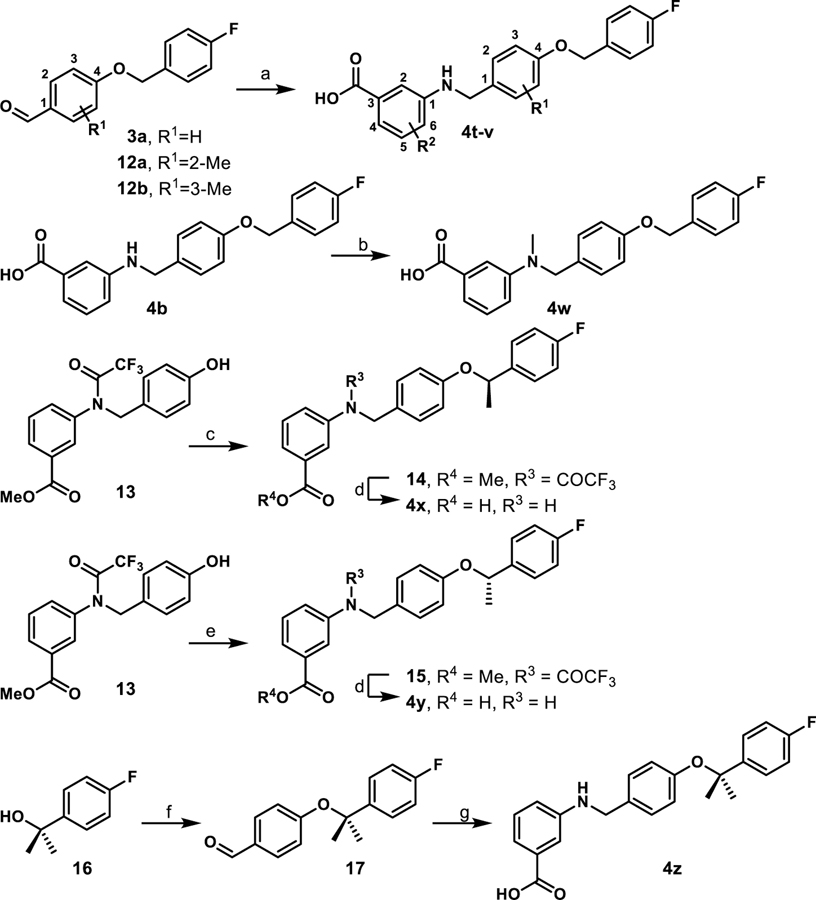
Scheme 3.
Synthesis of methylated derivatives 4t-za
aReagents and conditions: (a) i) 3-aminobenzoic acid or 3-amino-4-methylbenzoic acid, toluene, 130 °C, 2–3 h; ii) NaBH(OAc)3, acetic acid, THF, 25 °C, 12 h, 70–92%; (b) paraformaldehyde, NaBH(OAc)3, EtOH, reflux 2 h, 25 °C, 12 h, 43%; (c) (R)-1-(4-fluorophenyl)ethan-1-ol, di-tert-butyl azodicarboxylate, PPh3, toluene; (d) LiOH·H2O, THF/CH3OH/H2O, 25 °C, 12 h, 65–75%.; (e) (S)-1-(4-fluorophenyl)ethan-1-ol, di-tert-butyl azodicarboxylate, PPh3, toluene; f) 4-hydroxybenzaldehyde, TFAA, DBU, CuCl2, MeCN, 8%; (g) i) 3-aminobenzoic acid, toluene, 130 °C, ii) NaBH(OAc)3, AcOH, THF, 12 h, 25%. All yields reported are unoptimized.