Abstract
Indole is a microbiota metabolite that exerts anti-inflammatory responses. However, the relevance of indole to human non-alcoholic fatty liver disease (NAFLD) is not clear. It also remains largely unknown whether and how indole acts to protect against NAFLD. The present study sought to examine the association between the circulating levels of indole and liver fat content in human subjects and explore the mechanisms underlying indole actions in mice with diet-induced NAFLD. In a cohort of 137 subjects, the circulating levels of indole were reversely correlated with body mass index. In addition, the circulating levels of indole in obese subjects were significantly lower than those in lean subjects and were accompanied with increased liver fat content. At the whole animal level, treatment of high-fat diet (HFD)-fed C57BL/6J with indole caused significant decreases in the severity of hepatic steatosis and inflammation. In cultured cells, indole treatment stimulated the expression of PFKFB3, a master regulatory gene of glycolysis, and suppressed macrophage proinflammatory activation in a PFKFB3-dependent manner. Moreover, myeloid cell-specific PFKFB3 disruption exacerbated the severity of HFD-induced hepatic steatosis and inflammation, and blunted the effect of indole on alleviating diet-induced NAFLD phenotype. CONCLUSIONS: Taken together, our results demonstrate that indole is relevant to human NAFLD, and is capable of alleviating diet-induced NAFLD phenotypes in mice in a myeloid cell PFKFB3-dependnet manner. Therefore, indole mimetic and/or macrophage-specific PFKFB3 activation may be the viable preventive and/or therapeutic approaches for inflammation-associated diseases including NAFLD.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is characterized by hepatic steatosis and progresses to non-alcoholic steatohepatitis (NASH) when the liver displays overt inflammatory damage (1, 2). As the advanced form of NAFLD, NASH is one of the most common causes of liver cirrhosis and hepatocellular carcinoma (2–4). Numerous epidemiological and/or clinical data have indicated that, on the one side, patients who have obesity and type 2 diabetes are more likely to develop hepatic steatosis and inflammation (5, 6). On the other side, patients with NAFLD commonly display manifestations of type 2 diabetes, such as dyslipidemia and insulin resistance. Given this, a better understanding of the pathogenesis of NAFLD is key to development of novel and improved preventive and/or therapeutic approaches for NAFLD and related human metabolic diseases.
The incidence of NAFLD in obese populations is increased by 7 to 10 folds relative to that in the general population (7). Because of this, obesity-related factors are thought to critically contribute to the pathogenesis of NAFLD. For instance, inflammation is shown to cause hepatic steatosis likely through increasing liver expression of genes for lipogenic enzymes such as acetyl-CoA carboxylase 1 (ACC1) and fatty acid synthase (FAS) and decreasing liver expression of genes for fatty acid oxidation including carnitine palmitoyltransferase 1a (CPT1a) (8, 9). In addition, obesity-associated inflammation is shown to serve as a “second hit” to exacerbate liver proinflammatory responses. Consequently, intrahepatic cells including hepatocytes and macrophages/Kupffer cells, secrete a number of proinflammatory mediator and fibrogenic factors to drive inflammatory damage and initiate fibrotic process (10, 11). As a critical component of inflammation, macrophages are shown to play an essential role in regulating functions of metabolic cells, e.g., hepatocytes and adipocytes, thereby contributing to or protecting against the development and progression of hepatic steatosis and systemic insulin resistance depending on the status of macrophage activation (12–14). Because of this, there is an urgent need to understand better how macrophage activation status is regulated in the context of the pathogenesis of NAFLD.
Over the past decade, growing evidence has implicated roles for dysbiosis and microbiota-host interactions in the pathogenesis of NAFLD (15–18). For instance, microbiota alters intestine absorption of nutrients and intestine permeability (19–21). This, in turn, enhances the delivery of nutrients, microbiota-metabolites, and endotoxin to the liver and trigger or exacerbate hepatic fat deposition and inflammation (19, 22). As a microbiota metabolite generated by intestinal bacteria upon degradation of tryptophan, indole exerts powerful anti-inflammatory effects on several types of immune cells including macrophages (23–25). Recent evidence also suggests that the levels of indole-3-acetate (I3A), a derivative of indole, in both the liver and cecum of high-fat diet (HFD)-fed mice, a model of obesity-associated NAFLD, were decreased relative to those in control mice (26). At the cellular levels, supplementation of I3A decreased palmitate-induced lipogenic gene expression in AML12 hepatocytes and suppressed proinflammatory cytokine expression in RAW264.7 macrophages (26). However, it remains largely unknown whether and how indole exerts a protective role in NAFLD. In the present study, we explored how indole interacts with hepatocytes and macrophages to bring about anti-NAFLD therapeutic effect. Considering a possible link between indole and PFKFB3 (27), a master regulatory gene of glycolysis, we also examined the extent to which the PFKFB3 in myeloid cells is involved in indole actions on diet-induced NAFLD phenotype in mice.
MATERIALS AND METHODS
Human subjects and pertinent assays
A cohort of 137 Chinese subjects (aged 20 to 80 years), were included in this study, which included 75 lean, 51 overweight, and 11 obese male and female subjects. Body mass index (BMI) was calculated by dividing the weight by the height squared. Liver fat content was assessed by a 64-section multi-detector Computed Tomography (CT) scanner (Lightspeed, GE Healthcare, Milwaukee, WI, USA) as reported (28). Details are provided in SI. The study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University.
Animal experiments
Wild-type (WT) C57BL/6J and LysMCre+ mice, in which the expression of Cre recombinase is under the control of Lysosome M promoter, were obtained from Jackson Laboratory (Bar Harbor, ME). PFKFB3-floxed (PFKFB3F/F) mice were bred with LysMCre+ mice to generate myeloid cell-specific PFKFB3-disrupted (Mye-PFKFB3–/– or Mye-PFKFB3+/–) mice and littermate (Mye-PFKFB3+/+) control. Mice were maintained on 12:12-h light-dark cycles (lights on at 06:00) and subjected to studies detailed in Supplemental Information (SI). All diets are products of Research Diets, Inc (New Brunswick, NJ). All study protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Texas A&M University.
Histological, biochemical and molecular assays
Paraffin-embedded liver sections (4–5 μm) were used for histological and immunohistochemical assays. Plasma parameters were measured using metabolic assay kits and ELISA kits. Also, tissue and/or cell samples were prepared for selected assays including Western blots analysis, real-time PCR, and other assays detailed in SI.
Cell culture and treatment
Bone marrow cells from Mye-PFKFB3–/–, Mye-PFKFB3+/–, and Mye-PFKFB3+/+ mice were differentiated into macrophages (BMDM) (13, 14). After differentiation, BMDM were subjected to metabolic and inflammatory assays described in SI. Some BMDM were trypsinized and added to primary mouse hepatocytes isolated from free-fed WT C57BL/6J mice at a 1:10 ratio for co-culture studies (13). Details were provided in SI.
Statistical Methods
Numeric data are presented as means ± SEM (standard error). Statistical significance was determined using unpaired, two-tailed ANOVA or Student’s t tests. Differences were considered significant at the two-tailed P < 0.05.
RESULTS
Indole is relevant to obesity and hepatic fat content in human subjects
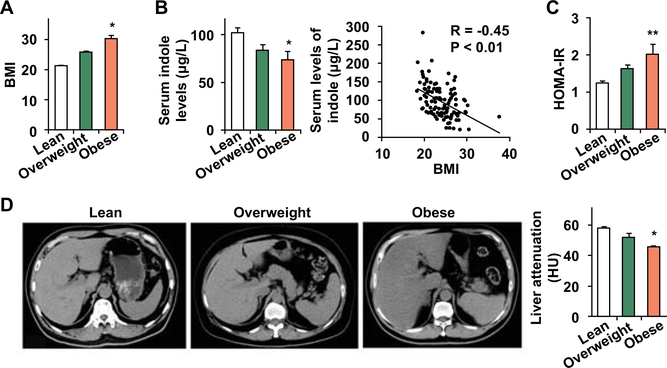
There is no published study addressing the relationship between indole and human NAFLD. In the present study, we included a cohort of 137 Chinese subjects comprising of 75 lean (BMI < 24 kg/m2), 51 overweight (BMI ≥ 24 kg/m2), and 11 obese (BMI ≥ 28 kg/m2) subjects. The levels of circulating indole were examined and associated with several biomarkers of obesity and hepatic fat content. As expected, the BMI of obese subjects was significantly higher than that of lean subjects (Fig. 1A). Similar to the data from the mouse study (27), obese human subjects displayed significant decreases in the circulating levels of indole (Fig. 1B). In all subjects, the circulating levels of indole were reversely correlated with BMI (Fig. 1B). Additionally, obese subjects revealed significantly higher numbers in HOMA-IR, indicating increased systemic insulin resistance (Fig. 1C). Among these subjects, liver attenuation calculated based on CT-scan images in obese subjects was significantly lower than that in lean subjects (Fig. 1D). Since a smaller number in liver attenuation reflects a greater degree of liver fat content (28), the decrease in liver attenuation in obese subjects indicated an increase in liver fat content relative to that in lean subjects. Together, these results verified a reverse correlation between indole and hepatic fat content, and suggest that indole appears to be relevant to human NAFLD.
Figure 1. Indole is relevant to human obesity and increased liver fat content.
The relevance of indole to human pathophysiology. (A) Body mass index (BMI). (B) Serum levels of indole and its correlation with BMI. (C) HOMA-IR was calculated using serum levels of insulin and glucose. (D) Hepatic fat content was estimated from liver attenuation (HU) based on CT-scan images. Left three panels, representative images; bar graph, quantitative data. A smaller HU indicates greater hepatic fat content. For A - D, the study included 75 lean, 51 overweight, and 11 obese subjects. Numeric are means ± SEM. *, P < 0.05 and **, P < 0.01 Obese vs Lean.
Indole treatment ameliorates HFD-induced hepatic steatosis and inflammation in mice
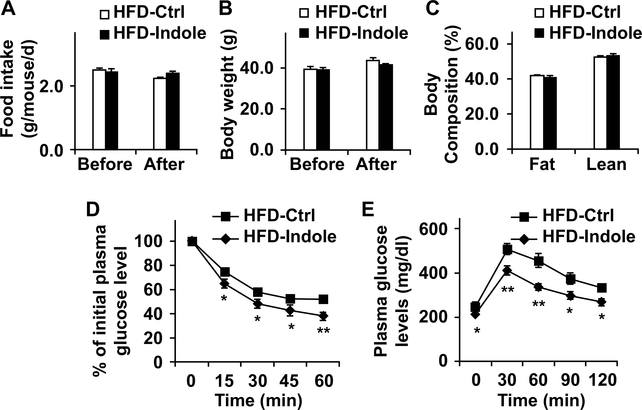
Dietary effects on gut microbiome, as well as its alteration by indole, are not well understood. We fed C57BL/6J mice an LFD or HFD for 12 weeks, and treated HFD-fed C57BL/6J mice with indole or control for the last 4 weeks of the feeding period. After feeding/treatment period, we analyzed microbiome in fecal samples of the mice. Compared with LFD, HFD feeding caused an increase in Bacteroidetes and a decrease in Firmicutes. Moreover, treatment with indole brought about changes in composition of gut microbiome in patterns similar to those of LFD-fed mice (Supplemental Fig. S1). This verifies that indole links gut microbiome and diet-induced NAFLD. Next, we analyzed indole kinetics and showed that indole was present in the liver at the levels much higher than those in the circulation within 4 hr after an oral indole dosing (Supplemental Fig. S2). We then sought to determine a direct role for indole in altering NAFLD phenotype. During the feeding/treatment period, HFD-fed and indole-treated (HFD-Indole) mice and control (HFD-Ctrl) mice consumed comparable amount of food (Fig. 2A). In addition, body weight and body composition of HFD-Indole mice did not differ significantly from their respective levels of HFD-Ctrl mice after the feeding/treatment period (Fig. 2B,C). However, HFD-Indole mice displayed significant decreases in the severity of systemic insulin resistance and glucose intolerance compared with HFD-Ctrl mice; as this was indicated by the results of inulin tolerance tests (ITT) and glucose tolerance tests (GTT) (Fig. 2D,E). These results demonstrate that indole treatment improves diet-induced systemic insulin resistance and glucose tolerance.
Figure 2. Indole supplementation ameliorates diet-induced insulin resistance and glucose intolerance.
Male C57BL/6J mice, at 5 – 6 weeks of age, were fed an HFD for 12 weeks. HFD-fed mice were also treated with indole (orally, 50 mg/kg, suspension in 5% bovine serum albumin (BSA)) or control (Ctrl, 5% BSA) daily for the last 4 weeks of HFD feeding. (A) Food intake was culaculaed for mice before and after indole or control tremant during the HFD feeding period. (B) Body weight was recorded before and after indole treatment. (C) Body composition was examined after the feeding/treatment period. (D,E) Insulin (D) and glucose (E) tolerance tests. After the feeding/treatment period, mice were fasted for 4 hr and given a bolus intraperitoneal injection of insulin (1 U/kg) (D) or glucose (2 g/kg) (E). For A - E, data are means ± SEM. n = 10 −12. *, P < 0.05 and **, P < 0.01 HFD-Indole vs. HFD-Ctrl for the same time point (in D and E).
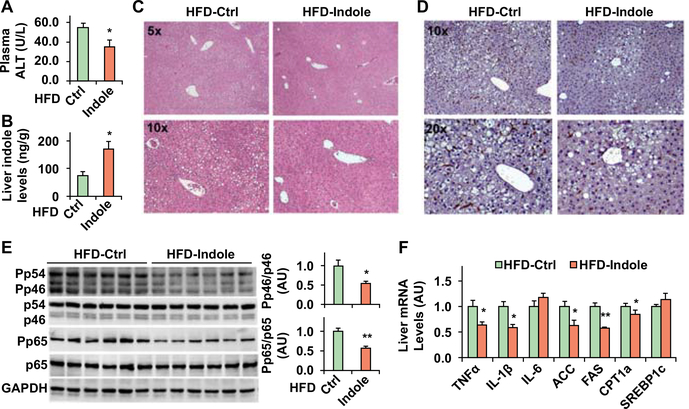
Next, we analyzed NAFLD phenotype. Compared with HFD-Ctrl mice, HFD-Indole mice displayed a significant decrease in plasma levels of alanine transaminase (ALT) and a significant increase in hepatic levels of indole (Fig. 3A,B); although showing no difference in liver weight (not shown). Indicated by the results of H&E staining of liver sections, HFD-Indole mice revealed a significant decrease in the severity of hepatic steatosis compared with HFD-Ctrl mice (Fig. 3C). HFD-Indole mice also displayed significantly decreased mRNAs of ACC and FAS; although CPT1a mRNAs were also decreased (Fig. 3F). When inflammation was analyzed, HFD-Indole mice revealed a significant decrease in the severity of HFD-induced liver inflammation, indicated by decreased numbers of liver macrophages/Kupffer cells, phosphorylation states of JNK p46 and NFκB p65, and mRNA levels of TNFα and IL-1β (Fig. 3D-F). Also, the anti-NAFLD effects of indole were comparable with those of metformin (Supplemental Fig. S3). Taken together, these results indicated that indole treatment alleviates NAFLD phenotype.
Figure 3. Indole treatment alleviates HFD-induced NAFLD phenotype in mice.
Male C57BL/6J mice, at 5 – 6 weeks of age, were fed an HFD for 12 weeks. HFD-fed mice were also treated with indole (orally, 50 mg/kg) or control (Ctrl) daily for the last 4 weeks of HFD feeding. (A) Plasma levles of ALT. (B) Liver indole levels. (C,D) Liver sections were stained with H&E (C) or for F4/80 (D). (E) Liver lysates were examined for the phosphorylation states of JNK p46 and NFκB p65. Bar graphs, quantification of blots. (F) Liver gene expression was examined using real-time PCR. For A, B, E, and F, numeric data are means ± SEM. n = 10 – 12 (A) or 6 – 8 (B, E and F). *, P < 0.05 HFD-Indole vs. HFD-Ctrl (in A, B and E) for the same gene (in F).
Indole treatment decreases hepatocyte fat deposition and lipogenic gene expression
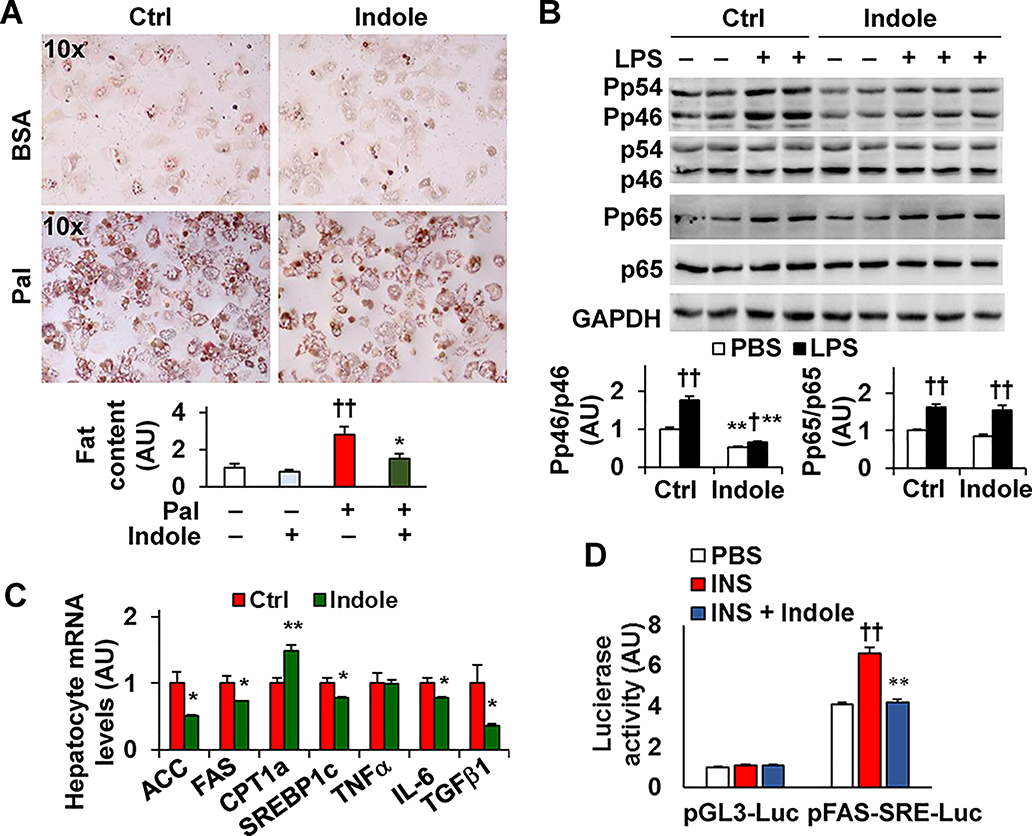
It has been shown that an indole derivate alters hepatocyte responses (26). Thus, we sought to examine the effect of indole on hepatocyte fat deposition and elucidate indole actions on lipogenic gene expression. In primary hepatocytes, treatment with indole caused decreases in palmitate-stimulated fat deposition and LPS-induced phosphorylation states of JNK p46 and NFκB p65 subunit (Fig. 4A,B). Additionally, indole treatment decreased hepatocyte mRNAs of ACC, FAS, and sterol regulatory element-binding protein 1c (SREBP1c). At the transcription level, indole treatment decreased the effect of insulin on stimulating SREBP1c transcription activity (Fig. 4C,D); although indole does not significantly alter SREBP1c maturation and/or translocation (data not shown). Considering that aryl hydrocarbon receptor (AhR) likely mediates indole actions, we also examined the extent to which AhR inhibition alters indole effects on hepatocytes. Upon treatment with an AhR inhibitor CH2232191, the effects of indole on decreasing hepatocyte fat deposition and proinflammatory responses were impaired (Supplemental Fig. S4). In addition, indole treatment improved hepatocyte inulin signaling (Supplemental Fig. S5), and promoted the generation of hepatocyte-derived factors that acted to decrease macrophage proinflammatory responses (Supplemental Fig. S6). These results indicate that indole actions on hepatocytes contribute to indole alleviation of NAFLD phenotype.
Figure 4. Indole exerts direct effects on hepatocytes.
(A) Hepatocyte fat deposition. Bar graph, quantificaiton of fat content. (B) Hepatocyte inflammatory signaling. Bar graphs, quantificatoin of blots. (C) Hepatocyte mRNAs. For A - C, primary hepatocytes were treated with indole (0.5 mM) or control (0.5% DMSO) for 24 hr. For A, cells were also incubated with palmitate (Pal, 250 μM) or BSA for 24 hr and stained with Oil Red O for the last 1 hr. For B, cells were treated with or without LPS (100 ng/mL) for the last 30 min. (D) Hepatocyte SREBP1c transcription activity. Cell were incubated in M199 in the absence of fetal bovine serum and transfected with a reporter construct in which the expression of luciferase is under the control of the SRE sequences on fatty acid synthase (pFAS-SRE-luc) or a control (pGL3-luc) for 24 hr, and incubated in the presence or absence of insulin (1 μM) and treated with or without indole (0.5 mM) for an additional 24 hr. For A - D, numeric data are means ± SEM. n = 4 – 6. *, P < 0.05 and **, P < 0.01 Indole vs. Ctrl in A, in B under the same condition, in C for the same gene, or INS + Indole vs. INS in D under the same condition; †, P < 0.05 and ††, P < 0.01 Pal vs. none in A or LPS vs. PBS with the same treatment in B or INS vs. PBS in D under the same condition.
Since indole is generated in the intestine, indole actions on enterocytes (intestinal epithelia cells, IEC) may produce IEC-derived factors that function to alter hepatocyte responses. To address this, we treated IEC with indole, and examined the effects of IEC factors on hepatocyte function. Compared with control, treatment with indole decreased IEC proinflammatory signaling. Moreover, hepatocytes incubated with conditioned media (CM) from palmitate- and indole-treated IEC revealed decreased hepatocyte fat deposition and proinflammatory responses compared with hepatocytes incubated with CM from palmitate- and control-treated IEC (Supplemental Fig. S7). This suggests that indole-driven IEC factors appear to alleviate hepatocyte events related to NAFLD.
Indole stimulates PFKFB3 expression and suppresses macrophage proinflammatory activation in an PFKFB3-dependent manner
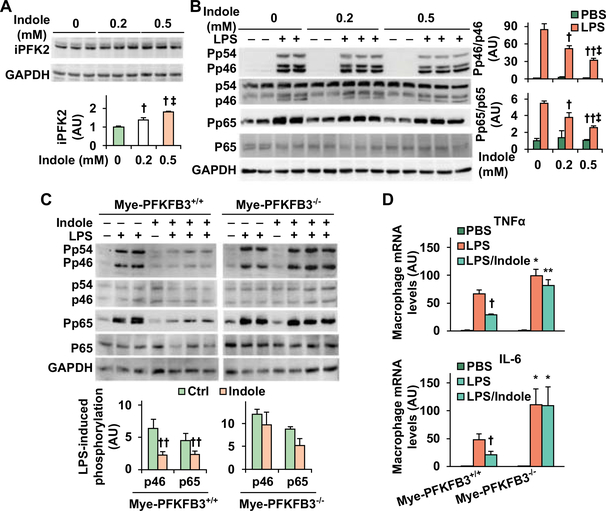
We previously showed a link between the proliferation of intestinal lactobacilli (27), which produces indole (29), and PFKFB3. Since PFKFB3 is expressed at high abundance in macrophages, but not hepatocytes, we sought to address whether indole alters PFKFB3 expression as it relates to macrophage activation status. Initially, we treated RAW264.7 cells with indole and examined the amount of iPFK2, the enzyme encoded by PFKFB3. Compared with control, indole treatment caused dose-dependent increases in iPFK2 (Fig. 5A). Consistent with the effect of indole on increasing iPFK2, treatment with indole increased the promoter activity of PFKFB3 in intestinal epithelial cells (Supplemental Fig. S8). Next, we examined the anti-inflammatory effect of indole. In RAW264.7 cells, treatment with indole significantly decreased LPS-induced phosphorylation states of JNK p46 and NFκB p65 in a dose-dependent manner (Fig. 5B). We then examined whether PFKFB3 is involved in indole actions on suppressing macrophage proinflammatory activation using macrophages differentiated from bone marrow cells of Mye-PFKFB3–/– mice (Supplemental Fig. S9) in which PFKFB3 was disrupted only in myeloid cells, and from bone marrow cells of control (Mye-PFKFB3+/+) mice. In Mye-PFKFB3+/+ BMDM, treatment with indole significantly decreased LPS-induced phosphorylation states of JNK1 p46 and NFκB p65, confirming the effect of indole on suppressing macrophage M1 activation. This effect, however, was abolished in Mye-PFKFB3–/– BMDM indole (Fig. 5C,D). Together, these results suggest that indole exerts a direct effect on suppressing macrophage M1 activation in a PFKFB3-dependent manner.
Figure 5. Indole stimatules PFKFB3 expression and suppresses macropahge proinflammatory actiaiton in a PFKFB3-dependent manner.
(A) Indole increases iPFK2 amount. (B) Indole suppresses macrophage proinflammatory signaling. For A and B, RAW264.7 cells were treated with or without indole (0.2 or 0.5 mM) for 24 hr in the absence or presence of LPS (100 ng/mL) for the last 30 min. (C,D) PFKFB3 disruption blunts the effect of indole on suppressing macrophag proinflammatory activation. Bone marrow cells were isolated from Mye-PFKFB3–/– and Mye-PFKFB3+/+ mice and differentiated into macrophages (BMDM). After differentiation, BMDM were treated with indole (0.5 mM) for 24 hr in the absence or presence of LPS (100 ng/mL) for the last 30 min (C) or LPS (20 ng/mL) for the last 6 hr (D). For A - C, cell lysates were subjected to Western blot analysis. Bar graphs, quantification of blots. For A - D, numeric data are means ± SEM. n = 4 – 6. *, P < 0.05 and **, P < 0.01 Mye-PFKFB3–/– vs. Mye-PFKFB3+/+ with the same treatment (LPS or LPS/Indole) (in D); †, P < 0.05 and ††, P < 0.01 Indole vs. Ctrl (in the absence of indole) (in A) under LPS-treated condition (in B) for the same protein (in C) or LPS/Indole vs. LPS within the same genotype (in D); ‡, P < 0.05 Indole (0.5 mM) vs. Indole (0.2 mM) (in A) under LPS-treated condition (in B).
The anti-NAFLD effects of indole are impaired in mice lacking PFKFB3 in myeloid cells
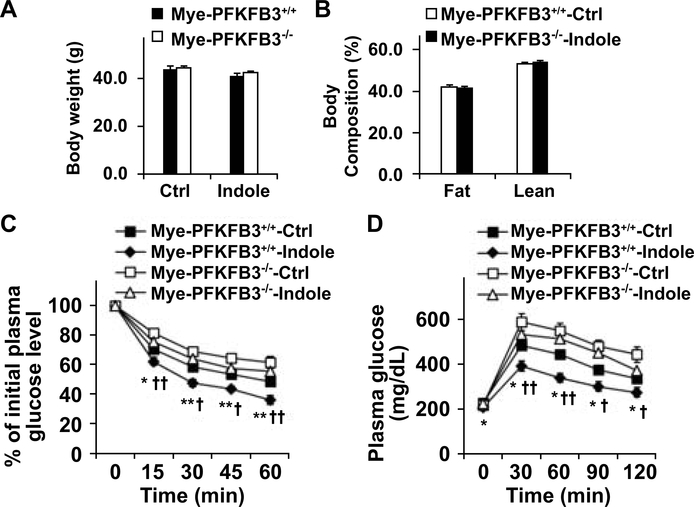
Since PFKFB3 disruption blunts the effect of indole on suppression of macrophage M1 activation, we sought to determine whether PFKFB3 disruption only in myeloid cells impairs the effect of indole on alleviating HFD-induced hepatic steatosis and inflammation. Initially, we verified that PFKFB3 disruption only in myeloid cells exacerbated HFD-induced NAFLD phenotype (Supplemental Figs. S10,S11). Next, we performed macrophage-hepatocyte co-cultures and confirmed that PFKFB3 disruption-driven macrophage factors promoted hepatocyte fat deposition and enhanced hepatocyte proinflammatory responses (Supplemental Fig. S12). We then treated HFD-fed Mye-PFKFB3–/– and Mye-PFKFB3+/+ mice with indole and analyzed NAFLD phenotypes of the selected mice. Indole treatment significantly decreased the severity of HFD-induced systemic insulin resistance and glucose intolerance without significantly altering food intake (not shown), body weight, and body composition of HFD-Mye-PFKFB3+/+ mice compared with control (Fig. 6A,B). However, the effects of indole on alleviating HFD-induced systemic insulin resistance and glucose intolerance were impaired in Mye-PFKFB3–/– mice.
Figure 6. Myeloid cell-specific PFKFB3 disruption impairs the effect of indole on improving HFD-induced systemic insulin resistance and glucose intolerance.
Male Mye-PFKFB3–/– and Mye-PFKFB3+/+ mice, at 5 – 6 weeks of age, were fed an HFD for 12 weeks and treated with indole (orally, 50 mg/kg) or control (Ctrl) daily for the last 4 weeks of HFD feeding. (A) Body weight was recorded at the end of feeding/treatment period. (B) Body composition was examined after the feeding/treatment period. (C,D) Insulin (C) and glucose (D) tolerance tests. Mice were fasted for 4 hr after the feeding/treatment period and received a bolus intraperitoneal injection of insulin (1 U/kg) or glucose (2 g/kg). For A - D, data are means ± SEM, n = 10 – 12. *, P < 0.05 and **, P < 0.01 Mye-PFKFB3+/+-Indole vs. Mye-PFKFB3–/–-Indole for the same time point (in C and D); †, P < 0.05 and ††, P < 0.01 Mye-PFKFB3+/+-Indole vs. Mye-PFKFB3+/+-Ctrl for the same time point (in C and D).
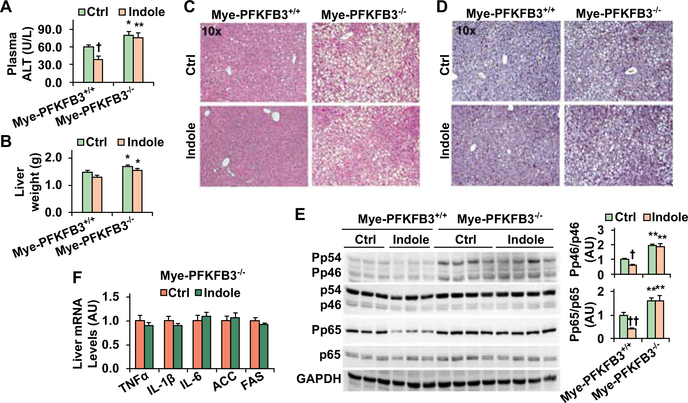
When NAFLD phenotype was analyzed, the levels of plasma ALT and liver weight in HFD-fed and control-treated Mye-PFKFB3–/– mice were significantly higher than their respective levels in HFD-fed and control-treated Mye-PFKFB3+/+ mice (Fig. 7A,B). Upon treatment with indole, HFD-fed Mye-PFKFB3+/+ mice, but not HFD-fed Mye-PFKFB3–/– mice, revealed a significant decrease in the levels of plasma ALT; although liver weight was not significantly altered in either HFD-fed Mye-PFKFB3–/– or Mye-PFKFB3+/+ mice. Additionally, indole treatment significantly decreased the severity of HFD-induced hepatic steatosis in Mye-PFKFB3+/+ mice (Fig. 7B). When liver inflammation was analyzed, indole treatment significantly decreased liver numbers of F4/80-positive cells (macrophages/Kupffer cells) and the phosphorylation states of JNK p46 and NFκB p65 in HFD-fed Mye-PFKFB3+/+ mice (Fig. 7C,D). These results confirmed the anti-NAFLD effect of indole, which, however, was significantly impaired in Mye-PFKFB3–/– mice (Fig. 7C-E). Specifically, in Mye-PFKFB3–/– mice indole treatment did not significantly decrease HFD-induced hepatic steatosis as did it in Mye-PFKFB3+/+ mice (Fig. 7C). Treatment with indole also did not decrease liver phosphorylation states of JNK p46 and NFκB p65 and mRNA levels of proinflammatory cytokines in HFD-fed Mye-PFKFB3–/– mice (Fig. 7E,F). Together, these results suggest that the PFKFB3 in macrophages is involved in the anti-NAFLD effect of indole.
Figure 7. The anti-NAFLD effects of indole was impaired in myeloid cell-specific PFKFB3 deficient mice.
Male Mye-PFKFB3–/– and Mye-PFKFB3+/+ mice, at 5 – 6 weeks of age, were fed an HFD for 12 weeks and treated with indole (orally, 50 mg/kg) or control (Ctrl) daily for the last 4 weeks of HFD feeding. (A) Plasma levels of ALT. (B) Liver weight. (C,D) Liver sections were stained with H&E (C) or for F4/80 (D). (E) Liver lysates were examined for proinflammatory signaling. Bar graphs, quantification of blots. (F) Liver gene was analyzed usig real-time PCR. For A, B, E, and F, numeric data are means ± SEM, n = 10 – 12 (A and B) and 6 – 8 (E and F). *, P < 0.05 and **, P < 0.01 Mye-PFKFB3–/– vs. Mye-PFKFB3+/+ under the same condition (Ctrl or Indole in A, B, and E); †, P < 0.05 and ††, P < 0.01 Indole vs. Ctrl within the same genotype (Mye-PFKFB3+/+ in E).
DISCUSSION
Indole is relevant to NAFLD pathophysiology and this notion originated from the link between microbiota and intestinal inflammation. The latter, together with increased intestine permeability and bacteria products, have been implicated to increase liver inflammation and dysregulate hepatic metabolism, thereby contributing to development and progression of NAFLD/NASH (30). While much evidence increasingly verifies the association between gut microbiota and NAFLD/NASH pathogenesis, it was the study by Park et al that validated a role for indole-3-carbinol (I3C), a derivate of indole, in amelioration of HFD-induced NAFLD phenotype in mice (31). A complementary study also indicated that the levels of I3A were decreased in livers of HFD-fed mice compared with control (26), suggesting a role for I3A in NAFLD phenotype. These findings led us to postulate a role for indole in NAFLD pathophysiology. Our postulation was also based on the findings that 1) hepatic indole levels were decreased in mice with diet-induced NAFLD and 2) indole exerts anti-inflammatory effects on macrophages whose increases in proinflammatory activation promote NAFLD phenotype (13, 14). Upon analyzing the circulating levels of indole in a cohort comprising of lean, overweight, and obese individuals, we validated our postulation. Notably, obese subjects revealed a significant decrease in the circulating levels of indole relative to lean subjects, which was accompanied with an increase hepatic fat content. In addition, among all subjects analyzed, the circulating levels of indole were reversely correlated with BMI. This indicates that decreased indole levels may account for increased fat deposition. As substantial evidence, treatment of HFD-fed mice with indole decreased hepatic fat deposition while increasing hepatic levels of indole (see below).
Indole exerts an anti-NAFLD effect and, in support of this indole treatment decreased the severity of HFD-induced hepatic steatosis, indicated by the results from H&E staining of liver sections and by the decreases in the expression of genes for lipogenesis. Indole treatment also decreased the severity of HFD-induced liver inflammation, indicated by decreased numbers of F4/80-postive cells in the liver, the levels of liver phosphorylation states of JNK p46 and NFκB p65, and the mRNAs of TNFα and IL-1β. Because indole treatment did not alter food intake, body weight, and fat mass of the mice, improvement in NAFLD phenotype in indole-treated mice is likely due to, in large part, indole actions on the liver. In in vitro studies, indole revealed direct effects on both hepatocytes and macrophages. Specifically, in hepatocytes, indole treatment decreased palmitate-induced fat deposition and LPS-stimulated proinflammatory responses, which recapitulated the in vivo findings. Since indole decreased hepatic lipogenic gene expression, we also explored the possible mechanisms and showed that indole treatment decreased the transcription activity of SREBP1c. As such, indole suppression of hepatic lipogenic events appears to contribute to the anti-NAFLD effect of indole. Consistent with its anti-inflammatory effects, indole treatment also decreased the proinflammatory responses in both hepatocytes and macrophages. These effects, certainly, contribute to the anti-NAFLD effect of indole.
It is a novel finding that indole stimulated the expression of PFKFB3 in macrophages, likely through increasing the transcription activity of PFKFB3 promoter. Based on this finding, we postulated and verified that indole stimulation of PFKFB3 is involved in the effect of indole on suppression of macrophage proinflammatory responses. In support of this, indole treatment caused a significant decrease in LPS-induced phosphorylation of states of JNK p46 and NFκB 65 in control macrophages. However, this effect of indole was nearly blunted in PFKFB3-disrupted macrophages. This finding not only points to a PFKFB3-invovled mechanism underlying the anti-inflammatory effect of indole, but also suggests that PFKFB3 acts to suppress macrophage proinflammatory activation. Previous studies suggest that increased glycolysis contributes to macrophage M1 activation. This was based on, in large part, the findings that LPS and/or hypoxia increase whereas treatment with 2-deoxyglucsoe (2DG) decreases macrophage glycolysis and M1 activation (32, 33). Considering that LPS and hypoxia also stimulate PFKFB3 expression (33), PFKFB3 is thought to promote macrophage M1 activation due to stimulating glycolysis (33). However, induction of PFKFB3 expression by LPS during macrophage M1 activation could be a defensive response because PFKFB3 disruption increases LPS-induced phosphorylation states of JNK1 p46 and NFκB p65 in bone marrow-derived macrophages as we reported here. Moreover, the anti-inflammatory role for PFKFB3 in macrophages is consistent with our previous findings in adipocytes and/or intestinal epithelial cells where PFKFB3 disruption or inhibition caused a compensatory increase in fatty acid oxidation and increased production of reactive oxygen species, thereby resulting in increased proinflammatory responses (34, 35). Also, recent findings have challenged the view concerning the role for glycolysis is promoting macrophage M1 phenotype. In particular, LPS stimulation also increases production of TCA metabolites such as succinate and citrate while stimulating glycolysis (36). In macrophages, succinate is an inflammatory signal (32). Additionally, 2DG not only decreases glycolysis, but also causes defects in TCA and appears to increase LPS-induced TNFα expression in macrophages. There also is evidence supporting that disruption of glycolytic flux functions as a signal to promote macrophage proinflammatory responses (37). Because of this, it appears that impaired TCA and aspartate-argininosuccinate shunt and increased pentose phosphate pathway, but not just increased glycolysis, are associated with macrophage M1 activation. As such, indole stimulation of PFKFB3 expression may work through complicated mechanisms involving alterations of various metabolic pathways to suppress macrophage proinflammatory activation.
The involvement of PFKFB3 in indole suppression of macrophage proinflammatory activation also led us to hypothesize that the PFKFB3 in macrophages is involved in the anti-NAFLD effect of indole. Initially, we validated the postulation that the PFKFB3 in macrophages has a protective role in development and progression of NAFLD; given that macrophages, at increased proinflammatory activation status, promote NAFLD (13, 14). As supporting evidence, myeloid cell-specific PFKFB3-disrupted mice revealed significantly increased severity of HFD-induced hepatic steatosis and inflammation. Moreover, the severity of HFD-induced NAFLD phenotype in Mye-PFKFB3–/– mice was greater than in Mye-PFKFB3+/– mice, suggesting a gene-dose effect of PFKFB3 disruption on exacerbating HFD-induced NAFLD phenotype. Upon co-culturing hepatocytes with PFKFB3-disrupted or control macrophages, we further validated that PFKFB3 disruption-derived macrophage factors promote palmitate-stimulated hepatocyte fat deposition and LPS-stimulated hepatocyte proinflammatory responses. These findings provided strong basis for us to define the role for PFKFB3 in myeloid cells in the anti-NAFLD effect of indole. As expected, in HFD-Mye-PFKFB3–/– mice, treatment with indole did not significantly decrease hepatic events as did it in HFD-control mice. Because PFKFB3 was disrupted only in myeloid cells, our findings convincingly indicate that PFKFB3 disruption impairs indole actions on suppressing macrophage generation of mediators that function to promote hepatocyte fat position and proinflammatory responses. Lastly, it should be pointed out that the finding that myeloid cell-specific PFKFB3 disruption nearly abolished the anti-NAFLD effect of indole does not necessarily downplay the contribution of indole reduction hepatocyte fat deposition to the anti-NAFLD effect of indole. This is because indole reduction of hepatocyte fat deposition is expected to decrease hepatocyte production of proinflammatory mediators, thereby relieving the effects of hepatocyte-derived mediators on enhancing macrophage proinflammatory activation. This view is substantiated by the results from macrophages incubated with conditioned media from indole-treated hepatocytes, and is worth further investigation. Indole-driven IEC factors also appear to alleviate hepatocyte alterations that are related to NAFLD. However, the extent to which hepatocyte responses brought about by indole-driven IEC factors contribute to indole alleviation of NAFLD phenotype needs to be further elucidated.
In summary, we showed, first the time, that the circulating levels of indole were decreased in obese individuals who also revealed increased liver fat content compared with those in lean subjects. Using mice with diet-induced obesity, we demonstrated that treatment with indole ameliorated diet-induced hepatic steatosis and inflammation. This anti-NAFLD effect was recapitulated in hepatocytes and macrophages upon treatment with indole. While validating a stimulatory effect of indole on macrophage expression of PFKFB3, we further demonstrated that disruption of PFKFB3 only in myeloid cells impaired the anti-NAFLD effect of indole. Therefore, we provided the primary evidence to validate the physiological relevance of indole to human obesity and hepatic steatosis, and support a protective role for indole in diet-induced NAFLD in manner involving the PFKFB3 in myeloid cells. Therefore, indole mimetic and/or macrophage-specific PFKFB3 activation may offer novel approaches for managing NAFLD.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in whole or in part by grants from the American Diabetes Association (1–17-IBS-145 to C.W.) and the National Institutes of Health (DK095862 to C.W.). C.W. is also supported by the Hatch Program of the National Institutes of Food and Agriculture (NIFA). Portions of this work were supported by the SRCS from the United States Department of Veteran’s Affairs to G.A. and NIH grants DK054811, DK076898, DK110035, DK115184, AA025157, and AA025997 to G.A, F.M., and S.G., and DK119421 to H.F.
Abbreviations
- AhR
aryl hydrocarbon receptor
- ACC1
acetyl-CoA carboxylase 1
- BMDM
bone marrow-derived macrophages
- BMI
body mass index
- BSA
bovine serum albumin
- CD
chow diet
- CPT1a
carnitine palmitoyl transferase 1a
- CT
computed tomography
- DBP
diastolic blood pressure
- FAS
fatty acid synthase
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GTT
glucose tolerance test
- H&E
hematoxylin and eosin
- HFD
high-fat diet
- LFD
low-fat diet
- I3A
indole-3-acetate
- I3C
indole-3-carbinol
- IL-1β
interleukin 1β
- IL-4
interleukin 4
- IL-6
interleukin 6
- IEC
intestinal epithelial cells
- iPFK2
inducible 6-phosphofructo-2-kinase
- IMDM
Iscove’s Modified Dulbecco’s medium
- ITT
insulin tolerance test
- LPS
lipopolysaccharide
- JNK
c-Jun N-terminal kinases
- NAFLD
non-alcoholic fatty liver disease
- NFκB
nuclear factor kappa B
- NASH
non-alcoholic steatohepatitis
- Pp46
phosphorylated JNK1 (p46)
- Pp65
phosphorylated p65 subunit of NFκB
- SBP
systolic blood pressure
- SREBP1c
sterol regulatory element-binding protein 1c
- TNFα
tumor necrosis factor α
- WC
waist circumference
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Sanyal AJ. Mechanisms of Disease: pathogenesis of nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol 2005;2:46–53. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 3.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, et al. Expanding the natural history of nonalcoholic steatohepatitis: From cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002;123:134–140. [DOI] [PubMed] [Google Scholar]
- 4.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 2010;51:1820–1832. [DOI] [PubMed] [Google Scholar]
- 5.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, et al. Association of nonalcoholic fatty liver disease with insulin resistance. The American Journal of Medicine 1999;107:450–455. [DOI] [PubMed] [Google Scholar]
- 6.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic Fatty Liver Disease: Predictors of Nonalcoholic Steatohepatitis and Liver Fibrosis in the Severely Obese. Gastroenterology 2001;121:91–100. [DOI] [PubMed] [Google Scholar]
- 7.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology 2006;43:S99–S112. [DOI] [PubMed] [Google Scholar]
- 8.Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee C-H. Adipocyte-derived Th2 cytokines and myeloid PPARd regulate macrophage polarization and insulin sensitivity. Cell Metab 2008;7:485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menghini R, Menini S, Amoruso R, Fiorentino L, Casagrande V, Marzano V, Tornei F, et al. Tissue inhibitor of metalloproteinase 3 deficiency causes hepatic steatosis and adipose tissue inflammation in mice. Gastroenterology 2009;136:663–672.e664. [DOI] [PubMed] [Google Scholar]
- 10.Napoli J, Prentice D, Niinami C, Bishop GA, Desmond P, McCaughan GW. Sequential increases in the intrahepatic expression of epidermal growth factor, basic fibroblast growth factor, and transforming growth factor β in a bile duct ligated rat model of cirrhosis. Hepatology 1997;26:624–633. [DOI] [PubMed] [Google Scholar]
- 11.Mormone E, Lu Y, Ge X, Fiel MI, Nieto N. Fibromodulin, an oxidative stress-sensitive proteoglycan, regulates the fibrogenic response to liver injury in mice. Gastroenterology 2012;142:612–621.e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saberi M, Woods N-B, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM, et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab 2009;10:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai Y, Li H, Liu M, Pei Y, Zheng J, Zhou J, Luo X, et al. Disruption of adenosine 2A receptor exacerbates NAFLD through increasing inflammatory responses and SREBP1c activity. Hepatology 2018;68:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo X, Li H, Ma L, Zhou J, Guo X, Woo S-L, Pei Y, et al. Expression of STING is increased in liver tissues from patients with NAFLD and promotes macrophage-mediated hepatic inflammation and fibrosis in mice. Gastroenterology 2018;155:1971–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumas M-E, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proceedings of the National Academy of Sciences of the United States of America 2006;103:12511–12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, et al. The severity of NAFLD is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology (Baltimore, Md.) 2016;63:764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, et al. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metabolism 2017;25:1054–1062.e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Chierico F, Nobili V, Vernocchi P, Russo A, Stefanis CD, Gnani D, Furlanello C, et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017;65:451–464. [DOI] [PubMed] [Google Scholar]
- 19.Nd Wit, Derrien M, Bosch-Vermeulen H, Oosterink E, Keshtkar S, Duval C, Bosch JdV-vd, et al. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. American Journal of Physiology-Gastrointestinal and Liver Physiology 2012;303:G589–G599. [DOI] [PubMed] [Google Scholar]
- 20.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008;57:1470–1481. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton MK, Boudry G, Lemay DG, Raybould HE. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. American Journal of Physiology - Gastrointestinal and Liver Physiology 2015;308:G840–G851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreznar JH, Keller MP, Traeger LL, Rabaglia ME, Schueler KL, Stapleton DS, Zhao W, et al. Host genotype and gut microbiome modulate insulin secretion and diet-induced metabolic phenotypes. Cell reports 2017;18:1739–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bansal T, Alaniz RC, Wood TK, Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proceedings of the National Academy of Sciences 2010;107:228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitfield-Cargile CM, Cohen ND, Chapkin RS, Weeks BR, Davidson LA, Goldsby JS, Hunt CL, et al. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes 2016;7:246–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cervantes-Barragan L, Chai JN, Tianero MD, DiLuccia B, Ahern PP, Merriman J, Cortez VS, et al. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, Sherr DH, Yarmush ML, et al. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Reports 2018;23:1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo X, Li H, Xu H, Halim V, Thomas LN, Woo S-L, Huo Y, et al. Disruption of inducible 6-phosphofructo-2-kinase impairs the suppressive effect of PPARg activation on diet-induced intestine inflammatory response. J Nutr Biochem 2013;24:770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hahn L, Reeder SB, Muñoz del Rio A, Pickhardt PJ. Longitudinal Changes in Liver Fat Content in Asymptomatic Adults: Hepatic Attenuation on Unenhanced CT as an Imaging Biomarker for Steatosis. AJR. American journal of roentgenology 2015;205:1167–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zelante T, Iannitti Rossana G, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013;39:372–385. [DOI] [PubMed] [Google Scholar]
- 30.Kolodziejczyk AA, Zheng D, Shibolet O, Elinav E. The role of the microbiome in NAFLD and NASH. EMBO molecular medicine 2019;11:e9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi Y, Yanagawa Y, Kim S, Park T. Involvement of SIRT1–AMPK signaling in the protective action of indole-3-carbinol against hepatic steatosis in mice fed a high-fat diet. The Journal of Nutritional Biochemistry 2013;24:1393–1400. [DOI] [PubMed] [Google Scholar]
- 32.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013;496:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tawakol A, Singh P, Mojena M, Pimentel-Santillana M, Emami H, MacNabb M, Rudd JHF, et al. HIF-1α and PFKFB3 mediate a tight relationship between pro-inflammatory activation and anaerobic metabolism in atherosclerotic macrophages. Arteriosclerosis, thrombosis, and vascular biology 2015;35:1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo X, Xu K, Zhang J, Li H, Zhang W, Wang H, Lange AJ, et al. Involvement of inducible 6-phosphofructo-2-kinase in the anti-diabetic effect of PPARg activation in mice. J Biol Chem 2010;285:23711–23720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Botchlett R, Li H, Guo X, Qi T, Zhao J, Zheng J, Woo S-L, et al. Glucose and palmitate differentially regulate PFKFB3/iPFK2 and inflammatory responses in mouse intestinal epithelial cells. Sci Rep 2016;6:28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haschemi A, Kosma P, Gille L, Evans Charles R, Burant Charles F, Starkl P, Knapp B, et al. The Sedoheptulose Kinase CARKL Directs Macrophage Polarization through Control of Glucose Metabolism. Cell Metabolism 2012;15:813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanman LE, Qian Y, Eisele NA, Ng TM, van der Linden WA, Monack DM, Weerapana E, et al. Disruption of glycolytic flux is a signal for inflammasome signaling and pyroptotic cell death. eLife 2016;5:e13663–e13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.