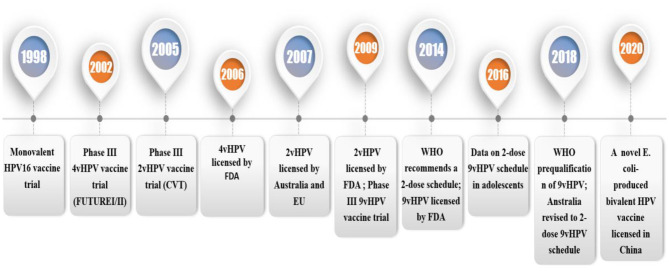
Figure 1.
Timeline of trials and licensure/registration of the HPV vaccines. 4vHPV, quadrivalent HPV vaccine; 2vHPV, bivalent HPV vaccine; 9vHPV, non-valent HPV vaccine; FDA, The U.S. Food and Drug Administration; HPV, human papillomavirus; EU, European Union; VLP, virus-like particle.1.1 HPV and HPV infection.