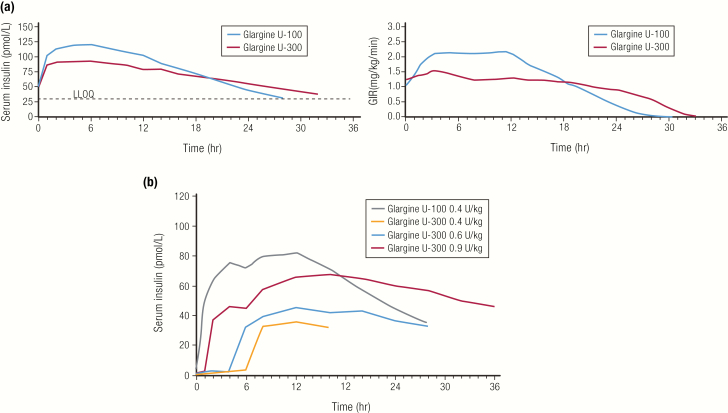
Figure 10.
Nonbioequivalence of U-100 and U-300 glargine. A: Comparison of PK and PD profiles for insulin glargine U-100 and insulin glargine U-300 (0.4 U/kg each) in a euglycemic clamp study at steady state in 18 patients with type 1 diabetes. Data from Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 Units/mL provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units/mL. Diabetes Care. 2015; 38(4):637–643. B: Dose dependence of PK profile of insulin glargine U-300 in a euglycemic clamp study in 24 patients with type 1 diabetes. Data from Center for Drug Evaluation and Research. Clinical Pharmacology Review: Toujeo® insulin glargine. 2014; https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/206538Orig1s000ClinPharmR.pdf. Accessed May 21, 2019.