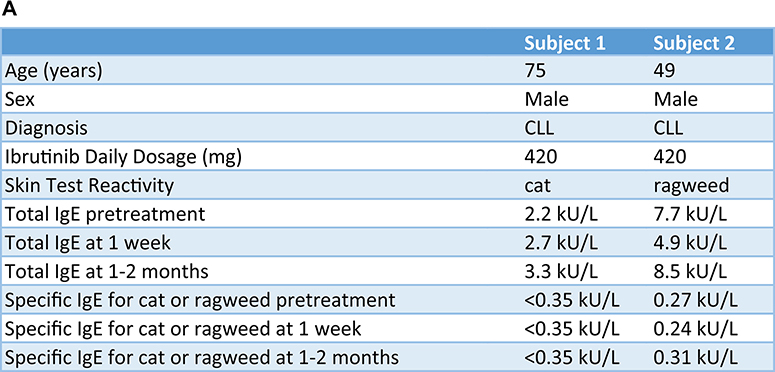
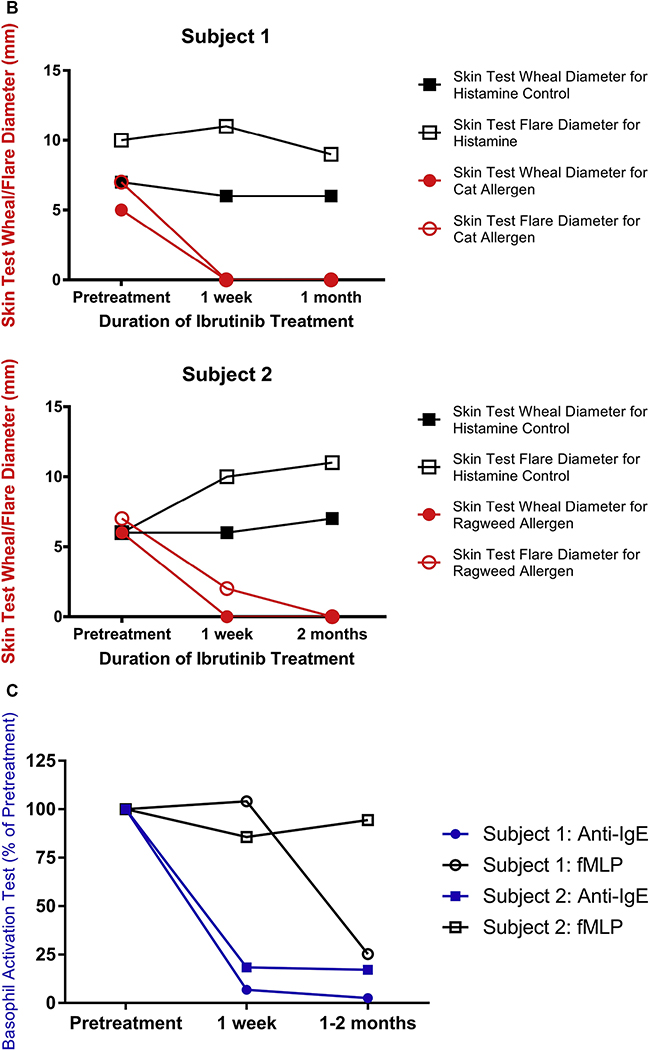
FIG 1.
Ibrutinib inhibits basophil and mast cell activation in 2 subjects prescribed ibrutinib for CLL treatment. A, Demographic characteristics, BTK inhibitor clinical indication, ibrutinib dose, and IgE levels are listed for each subject. B, Skin test responses to cat allergen (subject 1) and ragweed (subject 2) before and during ibrutinib treatment (420 mg daily) in 2 patients being treated for CLL. C, Basophil activation responses to anti-IgE (72% and 89% positive, respectively, before starting ibrutinib) or fMLP (12% and 16% positive, respectively, before starting ibrutinib) are shown for these same 2 subjects.