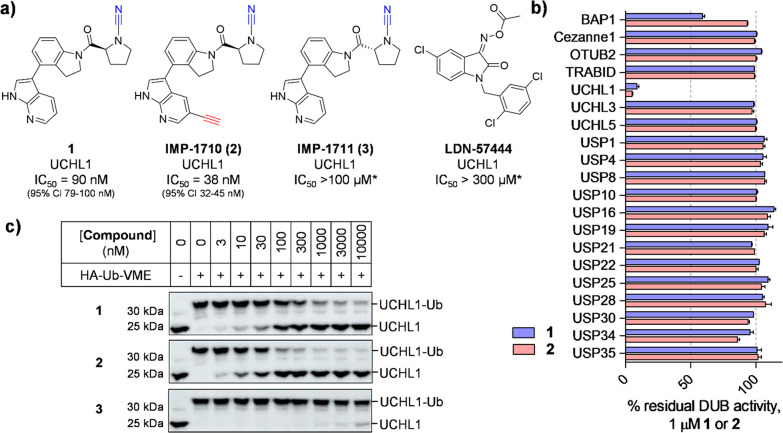
Figure 1.
Potency and selectivity of UCHL1 inhibitor (1), alkyne ABP (IMP-1710, 2), control compound (IMP-1711, 3), and LDN-57444. (a) Structures and UCHL1 IC50 values (Ub-Lys-TAMRA FP assay; *maximum assay concentration). (b) Selectivity profiling of 1 and 2 against DUBs in FP and FI assays (Ub-Lys-TAMRA and Ub-Rho110, respectively). See Supporting Information for 3 and LDN-57444 profiling. Data represent mean ± SEM (n = 2). (c) Immunoblot analysis of HA-Ub-VME UCHL1 labeling following HEK293T treatment with 1, 2, or 3 for 1 h. Dose-dependent competition for UCHL1 labeling occurs for active compounds, but not inactive enantiomer 3.