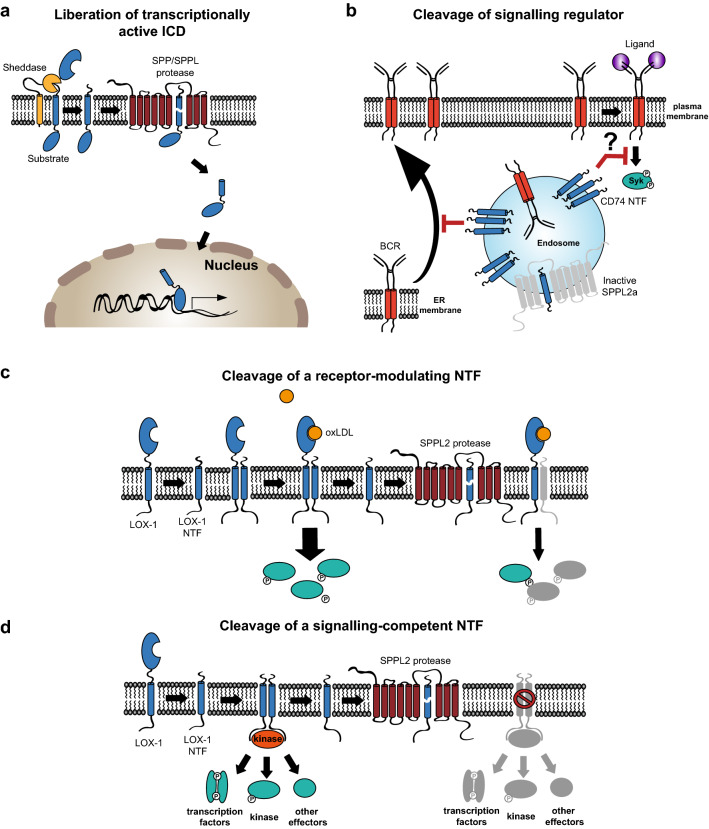
Fig. 2.
Mechanisms of signal transduction regulation by SPP/SPPL proteases. a SPP/SPPL proteases can transduce intracellular signals by releasing an intracellular domain (ICD) which either acts as transcription factor itself or is able to exert an impact on gene expression by interaction with the transcriptional machinery. b As exemplified by processing of CD74, SPP/SPPL proteases can cleave regulatory components of signalling pathways. The N-terminal fragment (NTF) of CD74 (depicted in blue) has the capability to influence subcellular trafficking of the B cell receptor (BCR, shown in red) and presumably also its downstream signalling. Since SPPL2a is required for clearance of the CD74 NTF, activity of this protease indirectly has a major impact on signal transduction in B cells. c, d SPP/SPPL proteases can also directly be involved in proteolytic processing of active receptor proteins like the Lectin-like oxidised low-density lipoprotein receptor 1 (LOX-1). In this case, the receptor NTFs can act as enhancers of the signaling of the full length receptor, which is induced by oxidised LDL (oxLDL) (c). Thus, turnover of the NTF controls LOX-1 signalling. Furthermore, the LOX-1 NTF was found to be an active signalling protein itself (d). In an autonomous way, most likely without the need of a ligand, this fragment activates MAP kinases. Therefore, the cellular levels of this fragment—controlled by SPP/SPPL proteases—are a direct determinant of the resulting signalling activation