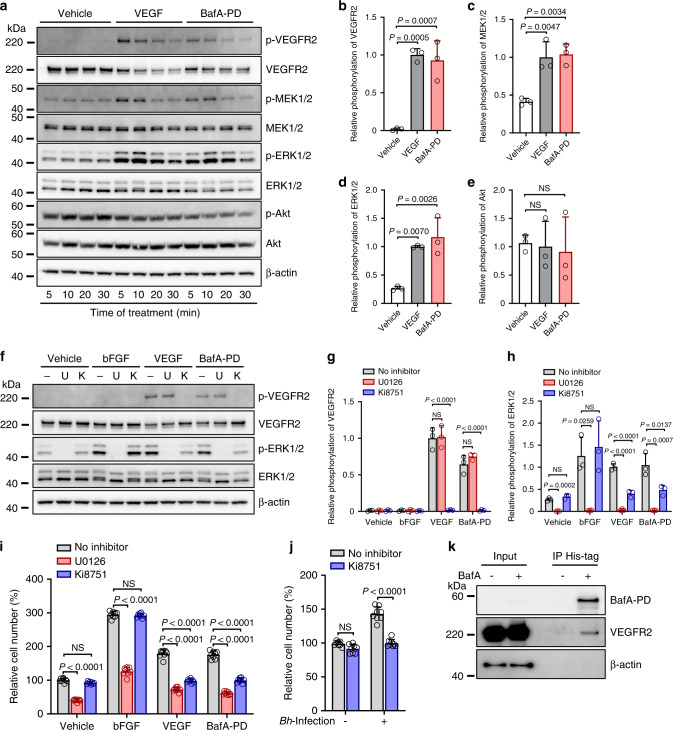
Fig. 4. BafA upregulates VEGFR2-ERK signaling pathway.
a–e BafA leads to phosphorylation of VEGFR2, MEK1/2, and ERK1/2 but not Akt. HUVECs were stimulated with VEGF (20 ng/mL) or BafA-PD (100 ng/mL) for indicated time, then subjected to immunoblotting using antibody against phosphorylated (p-) or total VEGFR2, MEK1/2, ERK1/2, Akt, or β-actin (a). Quantification of p-VEGFR2 (b), p-MEK1/2 (c), p-ERK1/2 (d) and p-Akt (e), represented as the ratio of phosphorylated to total proteins at 10 min (n = 3 biological replicates). Bars are colored based on the treatment: white for vehicle, gray for VEGF and red for BafA-PD (b–e). f–h MEK1/2 and VEGFR2 inhibitors suppress BafA-induced phosphorylation. − no inhibitor, U U0126 (MEK1/2 inhibitor), K Ki8751 (VEGFR2 inhibitor). Immunoblots (f), quantification of p-VEGFR2 (g), and p-ERK (h) are shown (n = 3 biological replicates). i MEK1/2 and VEGFR2 inhibitors suppress BafA-induced cell proliferation. HUVECs were treated with each inhibitor before stimulation with bFGF, VEGF, or BafA-PD. Three days after treatment, the cell numbers were counted (n = 8 biological replicates). j VEGFR2 inhibitor suppresses Bh-induced cell proliferation. HUVECs were treated with Ki8751 before Bh infection. Three days after infection, the cell numbers were counted (n = 8 biological replicates). k Co-immunoprecipitation (IP) shows interaction between BafA and VEGFR2. HUVECs were treated with or without BafA-PD, then BafA-PD was immunoprecipitated with anti-His-mAb-magnetic beads. Immunoblotting was performed to detect BafA and VEGFR2. β-actin served as a loading control for inputs. Similar results were obtained in three independent experiments. Data are mean ± s.d. (b–e, g–j). Statistical significance was tested using one-way ANOVA with Dunnett’s (b–e) or Tukey’s (g–j) multiple comparisons test. Source data are provided as a Source Data file.