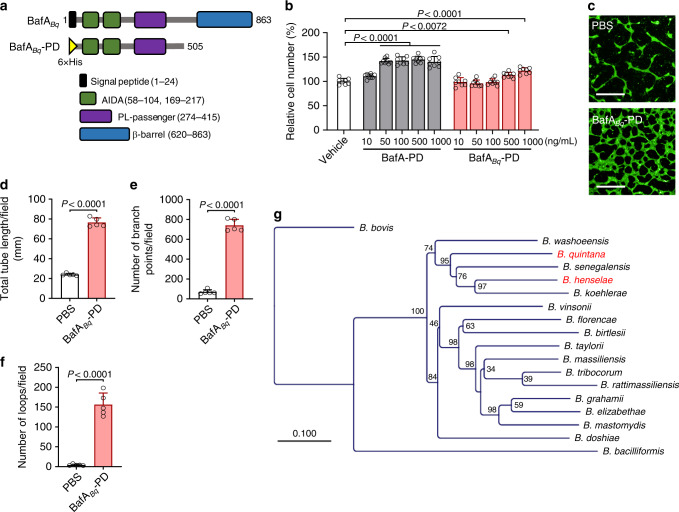
Fig. 5. The BafA homolog from B. quintana possesses in vitro angiogenic activity.
a Schematic representation of B. quintana-derived full-length BafA (BafABq) and the recombinant passenger domain (BafABq-PD). The full-length BafABq has a similar domain structure with the Bh-derived BafA. BafABq-PD was constructed as a His-tagged protein from the amino acid residues 25–505 of BafABq-PD. b Comparison of cell proliferation activities of BafABq-PD and BafA-PD (n = 8 biological replicates). c–f Tube formation assay. HUVECs sandwiched between collagen gels were incubated in the presence of PBS or BafABq-PD (300 ng/mL) for 24 h. The tube formation was imaged (c), and the total tube length (d), number of branch points (e), and number of loops (f) were calculated as described above. Scale bar = 500 µm (n = 6 biological replicates). Bars are colored based on the treatment: white for vehicle or PBS, gray for BafA-PD, and red for BafABq-PD (b–e). Data are mean ± s.d. (b, d–f). Statistical significance was tested using one-way ANOVA with Dunnett’s multiple comparisons test (b) or two-tailed unpaired student’s t test (d–f). g Phylogenetic tree of the BafA family proteins. The red letters indicate the species used in this study. Numbers at nodes indicate the percentage of bootstrap values. Source data are provided as a Source Data file.