Figure 2.
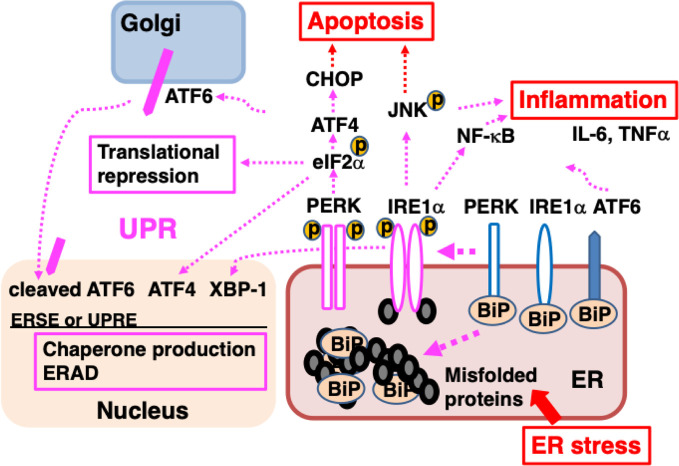
ER stress causes the accumulation of unfolded proteins in the ER, inducing the unfolded protein response (UPR). Misfolded proteins accumulate in the ER when protein folding is disturbed due to ER stress such as intrinsic defects (e.g., mutated sequences, missing subunits) or extrinsic injuries (e.g., ischemia, malnutrition, hypoxia, and toxicity). BiP dissociates from ATF6, IRE1α, and PERK, and associates with misfolded proteins; dissociation from BiP initiates the UPR. ATF6 is transported to the Golgi, where it is cleaved and the cytoplasmic portion of ATF6 is transported to the nucleus; there, it functions as a transcription factor, binding to ER stress response element (ERSE) and promoting the transcription of genes important for the UPR. After dissociating from BiP, IRE1α, and PERK multimerize and are activated by autophosphorylation. IRE1α is a type I membrane protein containing a serine/threonine kinase domain and an endoribonuclease domain at its cytoplasmic carboxyl terminus. Activated IRE1α splices XBP1 mRNA. The XBP1 protein binds to UPR elements (UPREs) in the transcriptional region of various genes required for the UPR and promotes their transcription. PERK is a serine/threonine kinase that phosphorylates and inactivates eIF2α, thereby halting the translation of most proteins. However, the translation of ATF4 is induced. ATF4 acts as a transcription factor. The UPR enhances the ability of cells to deal with increased levels of misfolded proteins through chaperone production, translational repression, and ER-associated protein degradation (ERAD). Activation of IRE1α leads to inflammation through the activation of MAP kinases (JNK) and NF-κB, inducing elevated expression of pro-inflammatory cytokines, such as TNFα and IL-6. Persistent ER stress induces the expression of CHOP, leading to apoptosis.