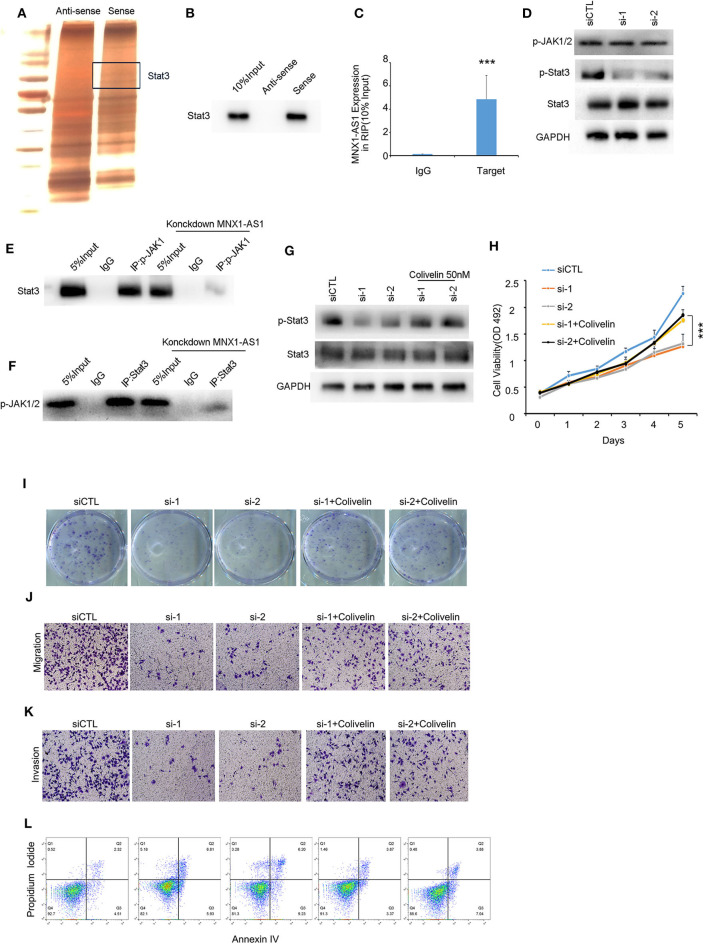
Figure 3.
MNX1-AS1 interacts State3 and promotes phosphorylation of Stat3 by enhance the interaction between p-JAK and Stat3. (A) Silver staining of proteins bound to MNX1-AS1. The RNA pull-down assay was performed with MDA-MB-231 cell lysates. A specific band was identified as Stat3 by mass spectrometry. (B) Stat3 interacted with MNX1-AS1 was confirmed by RNA pull-down assay and Western blot. (C) MNX1-AS1 interacted with Stat3 was confirmed by RNA immunoprecipitation (RIP). Bar graphs represent the mean ± SD of experimental triplicates. (D) Silencing MNX1-AS1 reduced phosphorylation of Stat3 but had no effect on phosphorylation of JAK1/2 in MDA-MB-231 cells, as indicated by Western blot. (E,F) Silencing MNX1-AS1 reduced the interaction between Stat3 and p-JAK1/2. (G) Phosphorylation of Stat3 reduced by silencing MNX1-AS1 was rescued by Stat agonist colivelin 50 nM. (H) Viability of MDA-MB-231 cell o reduced by silencing MNX1-AS1 was rescued by Stat agonist colivelin 50 nM. Bar graphs represent the mean ± SD of experimental triplicates. (I) Colony formation of MDA-MB-231 cell reduced by silencing MNX1-AS1 was rescued by Stat agonist colivelin 50 nM. (J) Migration of MDA-MB-231 cell reduced by silencing MNX1-AS1 was rescued by Stat agonist colivelin 50 nM. (K) Invasion of MDA-MB-231 cell reduced by silencing MNX1-AS1 was rescued by Stat agonist colivelin 50 nM. (L) Apoptosis of MDA-MB-231 cell induced by silencing MNX1-AS1 was rescued by Stat agonist colivelin 50 nM. ***P < 0.001.