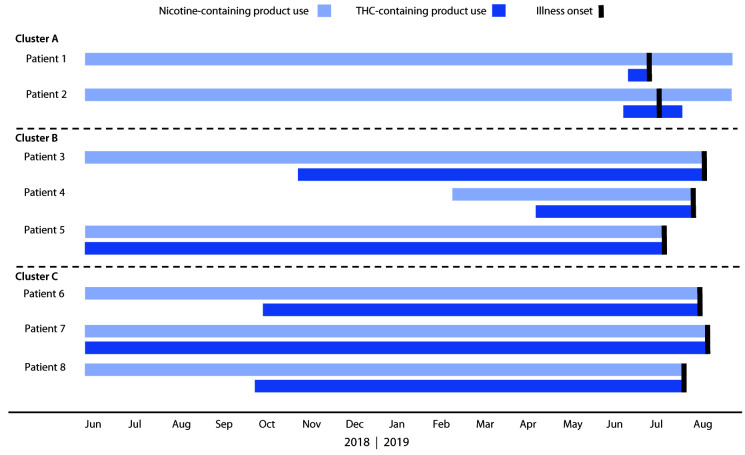
FIGURE 2.
Dates of initiation* and cessation of nicotine- and tetrahydrocannabinol (THC)-containing product use and illness onset among eight cluster-associated e-cigarette, or vaping, product use–associated lung injury patients† — Wisconsin, 2019
* All patients reported long-term use of nicotine-containing products, which were initiated a median of 33 months before symptom onset (range = 5–60 months).
† The following is a summary of pertinent events for patients in Cluster C; similar patterns of product use initiation, sharing, and symptom onset were observed for patients in clusters A and B. Within cluster C, patient 6 and patient 7 were close friends who reported frequent sharing of Dank Vapes, Chronic Carts, and various other illicit THC cartridges before symptom onset, which occurred for both patients in early July 2019. All of the THC cartridges used by patients 6 and 7 were obtained from the same local illicit dealer, from whom they had purchased similar THC cartridges for the past 9–12 months. In the week preceding symptom onset, they reported using more than the usual quantity together, approximately one half of a 1-g cartridge per person per day; they also reported daily use of nicotine-containing e-cigarette, or vaping, products. Patients 6 and 7 developed nausea, vomiting, fever, and respiratory symptoms within 5 days of each other and stopped using e-cigarette, or vaping, products shortly after symptom onset. Patient 8 was a friend of patients 6 and 7 but did not report sharing products with them and was unsure if they shared the same local illicit dealer. This patient also reported daily use of Dank Vapes, among other brands, beginning 9 months before symptom onset, which occurred 2 weeks before that of patient 6. All three patients were hospitalized in the intensive care unit, and one required mechanical ventilation. Bronchoalveolar lavage fluid from patient 6 tested positive for vitamin E acetate, and all three THC cartridges from patient 8 contained vitamin E acetate.