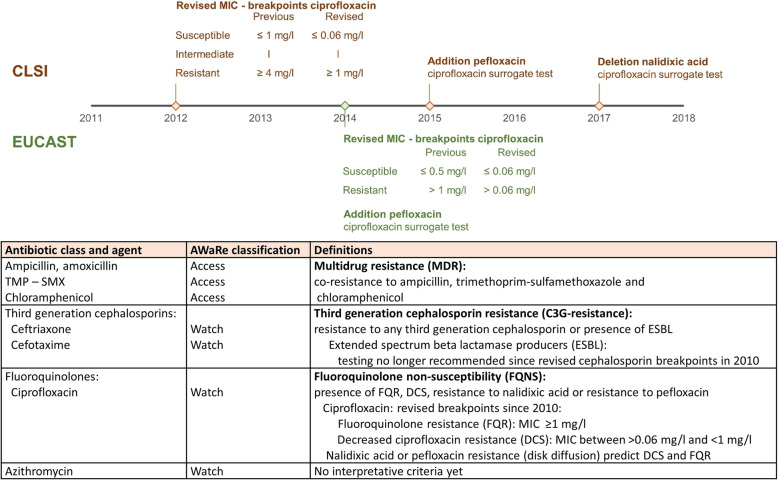
Fig. 1.
Overview of the definitions used in this review and of the changes in recommendations for fluoroquinolone susceptibility testing over time. A thin line represents the MIC—range of the intermediate susceptibility category according to CLSI (brown) [18, 19]. There is no intermediate ciprofloxacin susceptibility category defined by EUCAST (green) [22]. The antibiotic agents are classified according to antibiotic class and the AWaRe classification. The latter is defined in the WHO Essential Medicines List and categorizes antibiotics into Access, Watch, or Reserve group antibiotics [7, 8]. TMP–SMX, trimethoprim–sulfamethoxazole; MIC, minimum inhibitory concentration; CLSI, Clinical and Laboratory Standards Institute; EUCAST, European Committee on Antimicrobial Susceptibility Testing