Figure 1. TLR and RLR signaling pathways.
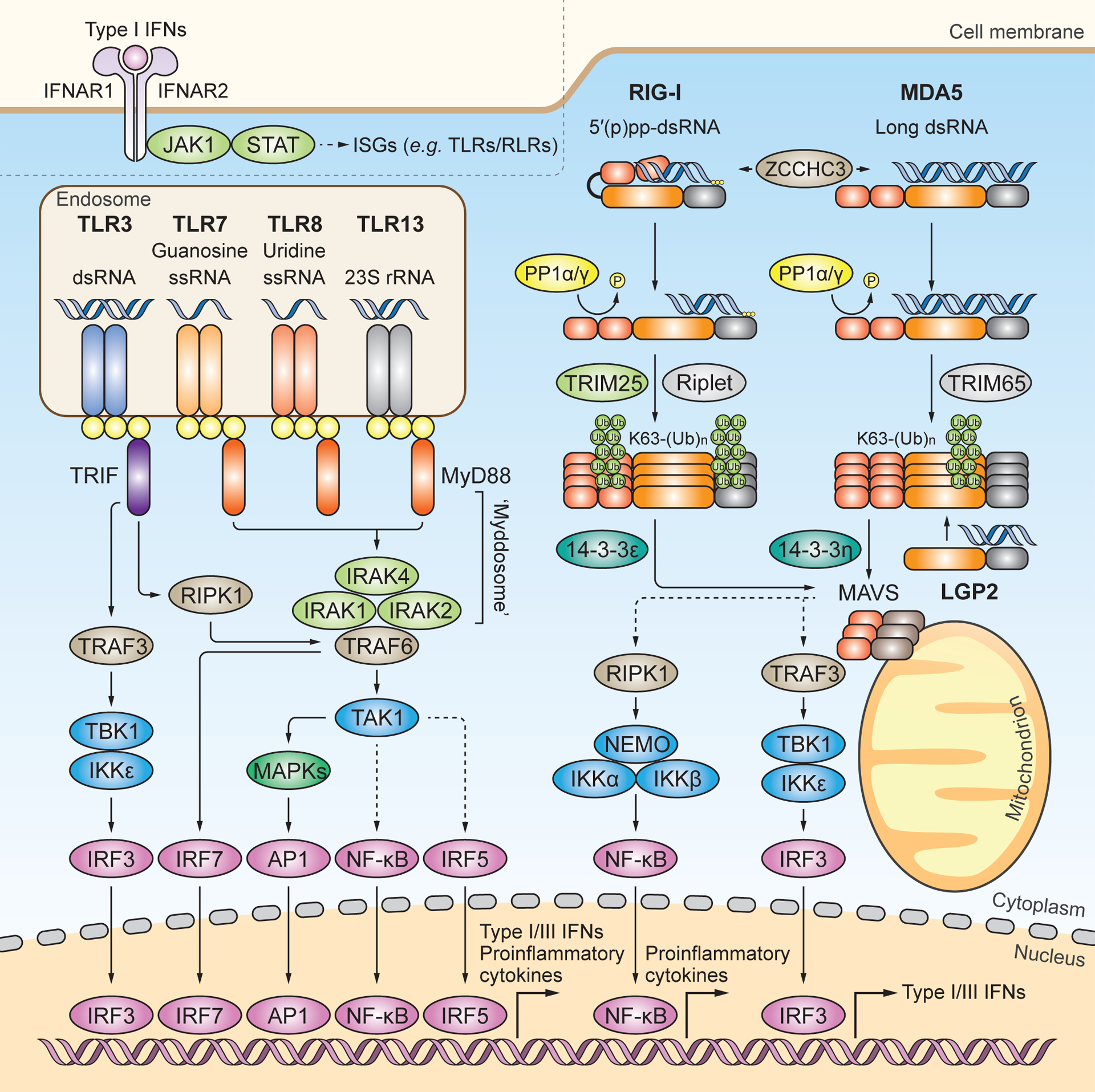
RNA-sensing TLRs predominately localize to the endosome and include TLR3, TLR7, TLR8 and TLR13. TLR3 recognizes dsRNA. TLR7 and TLR8 contain one binding site with specificity for guanosine and uridine, respectively, and the other binding site for ssRNA. TLR13 recognizes bacterial 23S rRNA. All RNA-sensing TLRs form homodimers upon activation. TLR7, TLR8 and TLR13 engage MyD88 as an adaptor protein to form the ‘Myddosome’, which is a multi-protein complex of MyD88, IRAK1, IRAK2 and IRAK4. The ‘Myddosome’ recruits TRAF6 and TAK1 to activate MAPKs, NF-κB, and IRF5. TLR3 signals through the adaptor protein TRIF to activate IRF3 via the TRAF3-TBK1–IKKε axis, or to activate MAPKs and NF-κB via the TRAF6-RIPK1-TAK1 axis. In plasmacytoid DCs, TLR7 activates IRF7 through TRAF6. It is noted that, for simplicity, this cell-type specific pathway is not illustrated separately. Nuclear translocation of activated transcription factors ultimately drives the expression of proinflammatory cytokines and type I and III IFNs. The RLR family members RIG-I and MDA5 localize to the cytoplasm and preferentially recognize 5’ di-or tri-phosphorylated short dsRNA and long dsRNA, respectively. ZCCHC3 facilitates RNA binding of RIG-I and MDA5. Structurally, RIG-I is held in an auto-repressed, closed conformation, while MDA5 likely adopts an open and flexible conformation. Cognate RNA-ligand binding induces conformational changes in RIG-I and MDA5 that expose their N-terminal CARDs, which are then subjected to posttranslational modifications (PTMs). PP1α or PP1γ-mediated dephosphorylation and TRIM25-mediated K63-linked ubiquitination at the CARDs induce further activation of RIG-I, which subsequently engages the chaperon protein 14–3-3ε for cytosol-to-mitochondrial translocation. Riplet modifies the C-terminal domain (CTD) of RIG-I with K63-linked-polyubiquitin chains. TRIM65 mediates K63-linked ubiquitination of MDA5 in its helicase domain, which induces MDA5 activation. The cytosol-to-mitochondria translocation of MDA5 is facilitated by 14–3-3η. Both RIG-I and MDA5 interact with and activate MAVS on mitochondria (and also other organelles), which subsequently activates IRF3 via TBK1 or IKKε and NF-κB via the IκB kinase (IKK) complex. LGP2 has been shown to facilitate MDA5 activation, whereas it inhibits antiviral signaling by RIG-I. Type I IFNs induced by TLR or RLR signaling bind to the type I IFN receptor (consisting of IFNAR1 and IFNAR2) and activate the Janus kinase (JAK)–signal transducer of activators of transcription (STAT) pathway to transcriptionally upregulate many ISGs including TLRs and RLRs themselves.
