Figure 3. RNA helicases, hnRNPs and ZBP1 as emerging RNA sensors and co-sensors.
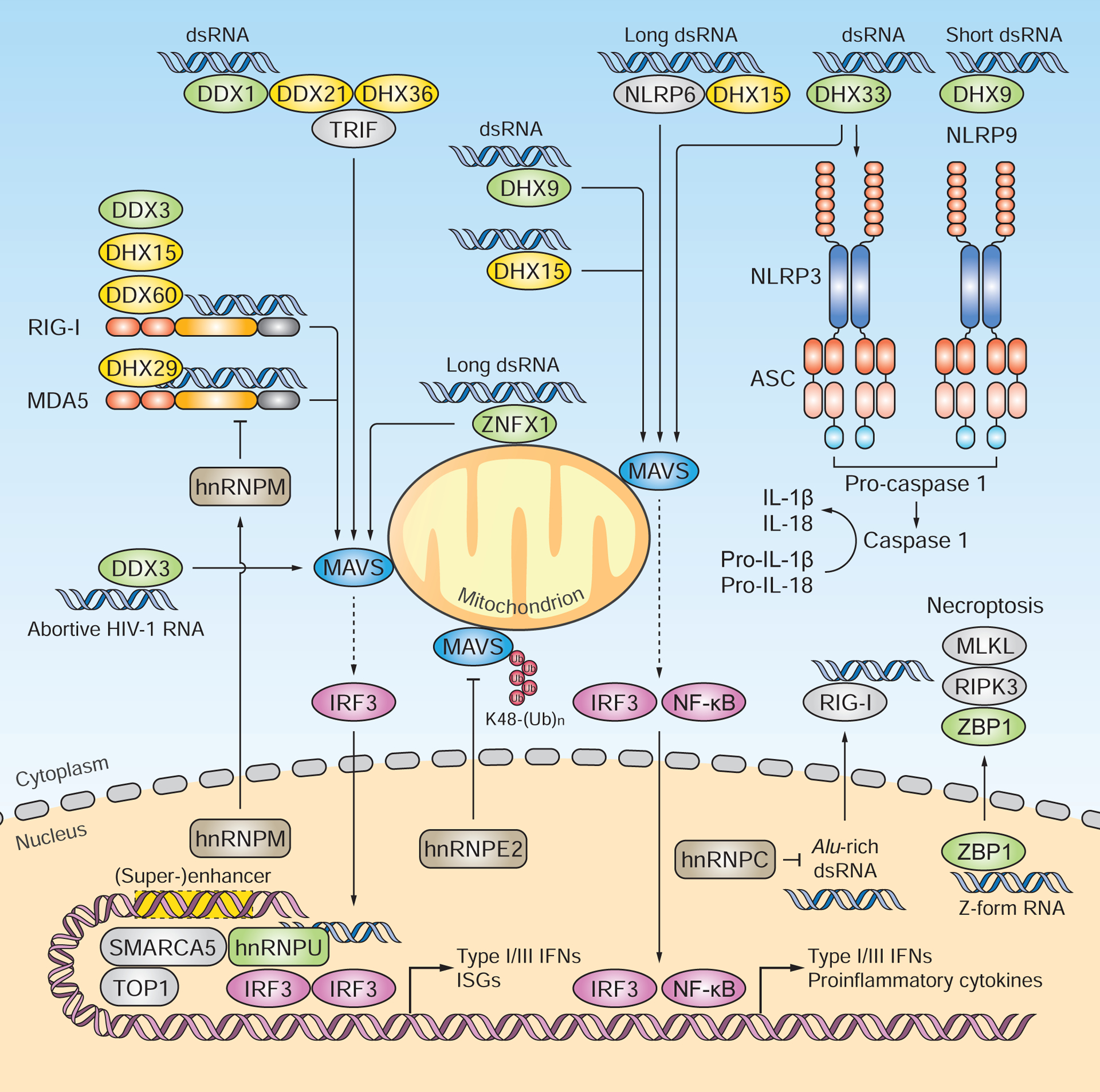
The DEAD-box or DEAH-box family proteins DDX1, DHX9 and DHX33 have been shown to sense RNA. DDX1, in complex with DDX21 and DHX36, recognizes poly(I:C) and induces type I IFN and proinflammatory responses via TRIF and MAVS. DHX9 associates with poly(I:C) and mediates MAVS-dependent cytokine responses in myeloid DCs. DHX9 also facilitates NLRP9b binding to short dsRNA [e.g. low-molecular-weight poly(I:C)] and thereby activates inflammasomes. DHX33 senses dsRNA [e.g. poly(I:C), reovirus genomic RNA, and RNase L cleavage products] and triggers NLRP3-mediated inflammasome activation and MAVS-dependent type I IFN induction. Multiple DEAD-box or DEAH-box helicases serve as RLR co-sensors. Most of these helicases (e.g. DDX3, DDX60, DHX15 and DHX29) interact with RLRs and potentiate their activation. DDX3 was found to also directly bind abortive HIV-1 RNA and to induce MAVS-dependent type I IFN responses. The hnRNP family members hnRNPE2, hnRNPM and hnRNPC negatively regulate RLR signaling. Upon virus-induced nucleus-to-cytoplasm translocation, HnRNPE2 interacts with MAVS and mediates its degradation. HnRNPM undergoes nucleus-to-cytoplasm redistribution and impairs the RNA-binding activity of RLRs. HnRNPC protects cells from accumulating RIG-I-stimulatory endogenous Alu-rich dsRNA. HnRNPU directly associates with HSV-1-derived dsRNA in the nucleus and, together with SMARCA5 and TOP1, activates the enhancers and super-enhancers that drive the transcription of antiviral and proinflammatory genes. ZBP1 senses both viral and host ‘Z-form’ dsRNA and then induces necroptosis through RIPK3 and MLKL. Green signifies proteins that are reported to function as direct RNA sensors; yellow indicates proteins that serve as RLR or NLR co-sensors.
