Figure 1. Schematic timeline of infection and inflammatory complications associated with CAR T-cell therapy.
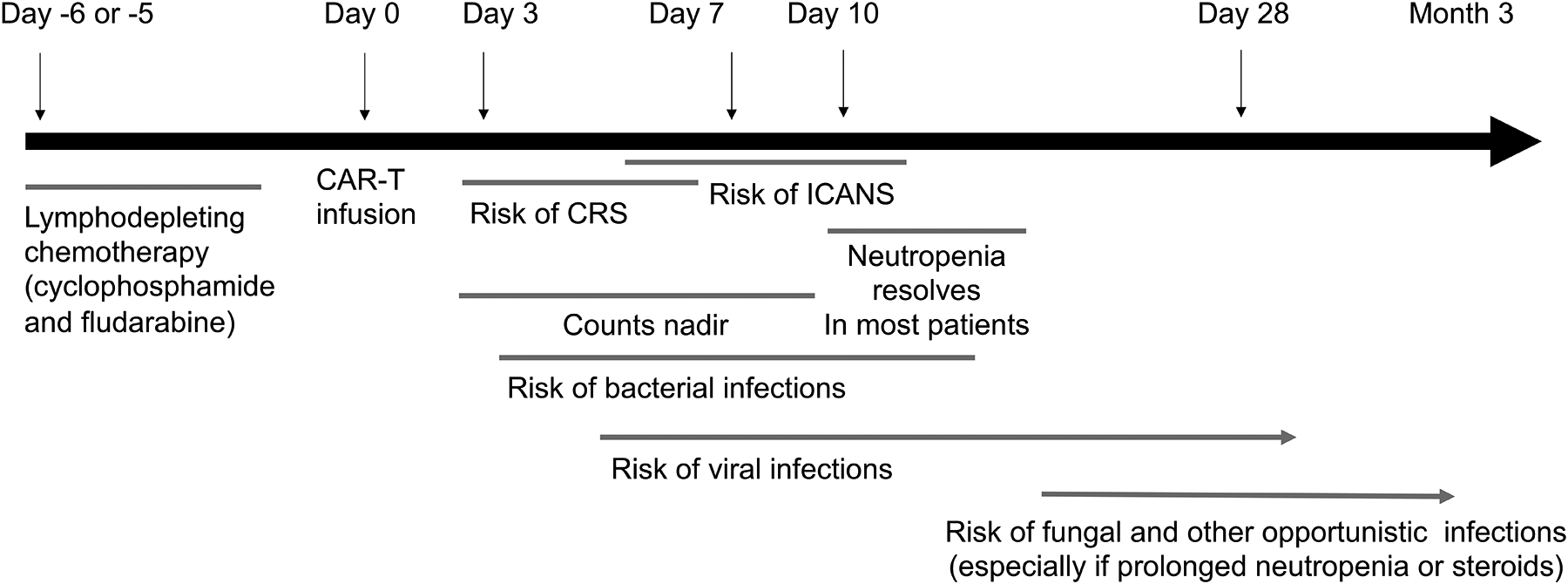
CAR T-cell therapy can be broken down into three phases: the week prior to infusion, when a preparative chemotherapy regimen is administered to achieve lymphodepletion prior to CAR T-cell infusion, which is known as Day 0. Not all protocols use chemotherapy, but many cancer protocols incorporate cyclophosphamide and fludarabine administered over 3–5 days, followed by a rest period of 1–2 days. During the first 2 weeks after CAR T-cell infusion, there is elevated risk of cytokine release syndrome (CRS) and immune-effector-cell associated neurotoxicity syndrome (ICANS), and this often coincides with the nadir of blood counts from the preparative regimen. Day 28 from CAR T-cell infusion usually marks a restaging evaluation and most (but not all) patients have achieved hematologic recovery. Host immunity is impaired during this period, and increased susceptibility to bacterial, viral, fungal and opportunistic infections can be see as a function of neutrophil decline (for bacteria and fungi) and T-cell and B-cell dysfunction, which can be multifactorial from prior therapies and the CAR T-cell target.
