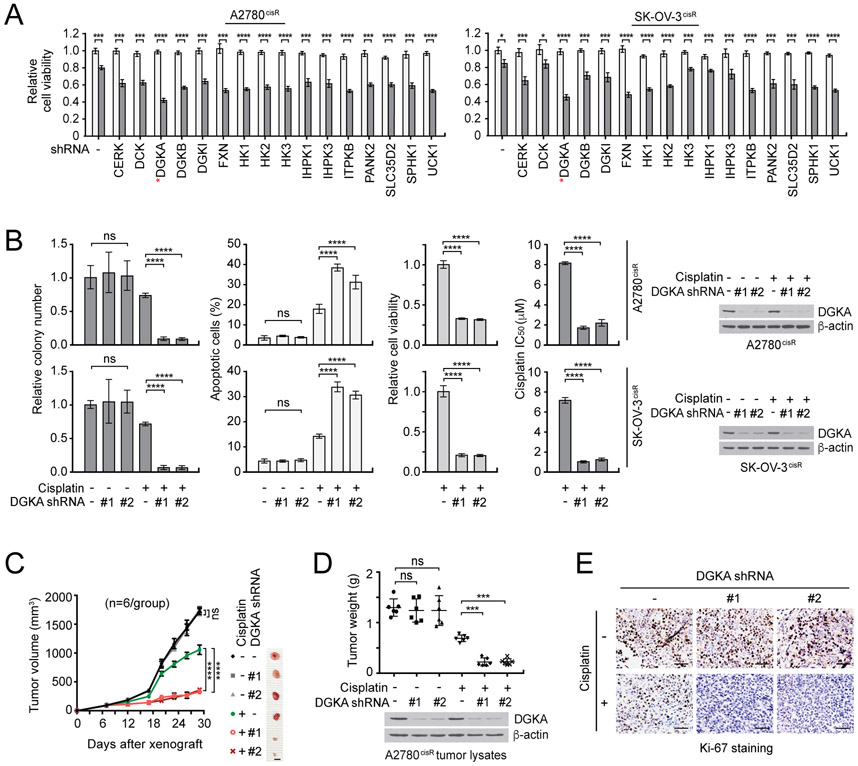
Figure 1. DGKA is identified as a critical cisplatin resistance driver in ovarian cancer.
A, Results of synthetic lethality screen targeting top 16 kinases acting on metabolites from a kinome shRNA library with cisplatin in cisplatin-resistant ovarian cancer cell lines. A2780cisR and SK-OV-3cisR cells were infected with pooled shRNA clones and sublethal doses of cisplatin (5 μg/ml A2780cisR and 2 μg/ml SK-OV-3cisR) for 48 hr. Cell viability was determined by CellTiter-Glo luminescent cell viability assay. White bars: no cisplatin treated; Gray bars: cisplatin treated. B, Colony formation potential, apoptotic cell death, cell viability, and cisplatin sensitivity (IC50) in ovarian cisR cancer cells with DGKA knockdown and cisplatin treatment for 48 or 72 hr. Stable DGKA knockdown cells were treated with cisplatin and viability was assessed as in (A). Apoptotic cells were assayed by annexin V staining. DGKA knockdown efficacy is shown by immunoblotting. C-E, Effect of DGKA loss on cisplatin sensitivity in xenograft mice. Mice bearing A2780cisR variants were treated with PBS or cisplatin (5 mg/kg/i.p. twice/week) from 7 days after tumor injection. i.p.: intraperitoneal injection. Tumor size (left), tumor weight (middle), and tumor proliferation rates assessed by Ki-67 staining (right). Scale bars represent 10 mm for (C)and 50 μm for (E). (A and B) n=3 technical replicates. Results of one representative experiment from two (A) and three (B) independent experiments are shown. (C-E) n=6. Error bars represent SEM for (C) and SD for all others. P values were determined by Student’s t-test for (A), one-way ANOVA for (B) and (D), and two-way ANOVA for (C) (ns: not significant; ***P < 0.001; ****P < 0.0001).