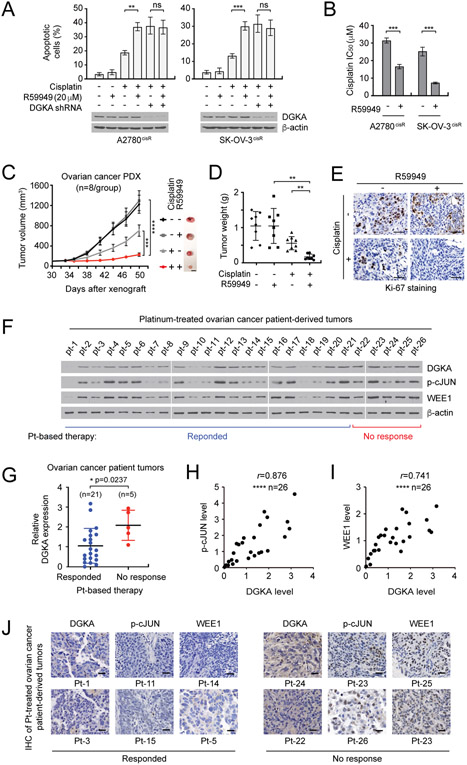
Figure 6. DGK inhibitor treatment sensitizes ovarian cancer cells to cisplatin treatment and DGKA-c-JUN-WEE1 signaling correlates with cisplatin resistance in ovarian cancer patient tumors.
A. Effect of DGK inhibitor R59949 on apoptotic cell death in cells with or without DGKA knockdown. B, Cisplatin response in cells treated with R59949 and cisplatin. C-E, Effect of R59949 and cisplatin on ovarian PDX tumor growth. Ovarian PDX mice were treated with cisplatin (5 mg/kg/i.p. twice/week) and R59949 (10 mg/kg/s.c. once every two days) after 30 days of xenograft. s.c.: subcutaneous injection. F, DGKA, p-c-JUN, and WEE1 levels in tumors derived from ovarian cancer patients who received platinum therapy were assessed by immunoblotting. Responded: patients who responded to platinum therapy (pt-1 ~ pt-21). No response: patients who had disease recurrence within 6 months after platinum therapy (pt-22 ~ pt-26). G, Relative DGKA expression in ‘Response’ and ‘No response’ groups. The immunoblots shown in (F) were quantified by ImageJ software. H and I, Correlation between DGKA and p-c-JUN levels (H) or DGKA and WEE1 levels (I) in tumor samples. J, Representative DGKA, p-c-JUN, and WEE1 IHC staining of platinum-treated ovarian cancer patient-derived tumors. Scale bars represent 10 mm (C), 50 μm (E), and 25 μm (J). (A and B) n=3 technical replicates and results of one representative experiment from three independent experiments are shown. (C-E) n=8. Results of one representative experiment from two independent experiments are shown for (F). (G-I) n=26. Error bars represent SEM for (C) and SD for all others. P values were determined by one-way ANOVA for (A) and (D), Student’s t test for (B) and (G), two-way ANOVA for (C), and Pearson correlation for (H) and (I) (ns: not significant; **P < 0.01; ***P < 0.001; ****P < 0.0001).