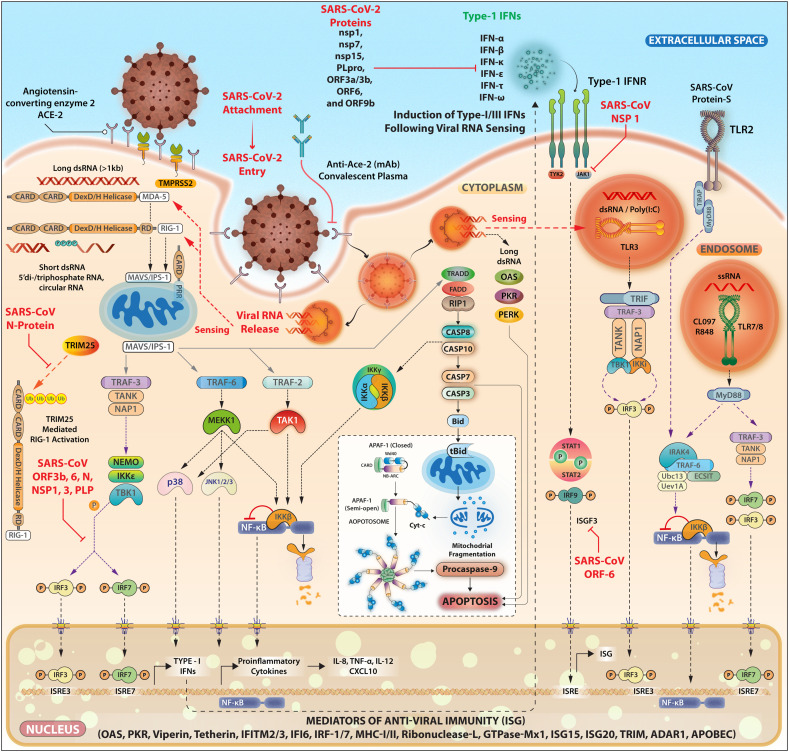
Fig. 2.
SARS-CoV-2 intracellular signaling and potential targets for immunoevasion and host cells particularly macrophages elicit a variety of antiviral immunity genes shown above.
The induction of TLR2 (via S protein), TLR3, TLR7, TLR8 (via virus-derived RNA following internalization process) leads to the expression of the pro-inflammatory mediators and IFNs through induction of the transcription factors NF-κB, IRF3, and IRF7. Viral-derived RNAs activate PKR- and OAS-related anti-viral pathways. The cytoplasmic sensors such as MAD-5 and RIG-1 also recognize various types of virus-derived RNAs and signal through adaptor MAVS/ISP-1 (located on our membrane of mitochondria). SARS-CoV-2 affects the cells of the innate immune system in particular monocytes, macrophages, and dendritic cells. These cells of the innate system play a crucial role in curbing the viral replication through the induction of Type-I IFNs assisted with the complement proteins and natural immunoglobulins against viral epitopes. These cellular responses (induction of proinflammatory mediators and IFNs Type-I, III) are tightly regulated by a series of intracellular signaling pathways elicited by surface receptors like TLRs, DC-SIGN, FcRs, and ACE-2 and TMPRSS2. Upon the viral entry into the host cells (via the clathrin-dependent internalization or ACE-2 mediated internalization) the spontaneous unloading of viral RNA is a subsequent step that follows. The viral RNA triggers the activation of intracellular RNA sensors like RIG-1 and MDA-5 each operating with distinct RNA conformations. RNA sensors then interact with MAVS that initiates Type-1 IFN signaling by activating the nuclear translocation of NF-κB and IRF3. The oligomeric RIG-1-CARD assembly and the polymeric formation of MAVS act as a signalosome for conducting the viral sensing signals further, which bifurcates into the activation of TRAF-2/6 to activate IKK complex and NF-κB activation. The other branch signals through TRAF-3 and activates the TANK/IKKγ/IKKε/TBK1 complex that acts as activators of IRF-3/7. Altogether the IRF-3/7 activation along with NF-κB drives the IFN and proinflammatory gene expression with the help of CREB-binding protein/p300 and transcription factors c-Jun and ATF-2. The IFN synthesized and secreted this way acts on the distant cells (paracrine) mode for spreading anti-viral immunity and in autocrine modes to fortify the intracellular viral clearance. SARS-CoV-2, however, attenuates these signaling pathways at various interception nodes. SARS-CoV proteins like ORF-9b may attenuate this antiviral response through targeting of MAVS by seizing poly (C)-binding protein 2 (PCBP2) and the HECT domain E3 ligase AIP4 to trigger the degradation of MAVS (not shown) along with TRAF-3 and TRAF-6. While ORF6 is reported to antagonize the STAT-1 function by sequestering its nuclear import factors. SARS-ORF-3b, ORF-6, and nucleocapsid protein function to antagonize interferon production. Besides this, the host mRNA destabilizing functions of NSP-1 are also reported. However, Nsp1 protein suppresses IFN-β mRNA accumulation without inhibiting IRF3 dimerization. Similarly, SARS-CoV NSP-15 inhibits MAVS-induced apoptosis sustaining the intracellular viral presence. SARS-CoV N protein can activate AP-1 but not the NF-κB signaling pathway. SARS-CoV proteins have been shown to inhibit the JAK-STAT pathway in the infected cell that responds to the Type-I IFNs secreted from bystander/neighboring cells. STAT-1-STAT-2-heterodimers combines with the IRF-9 to form the ISGF3 complex. This complex is crucial for the activation of genes harboring ISRE in their promoter regions. Viral protein ORF-6 blocks the nuclear import of ISGF3 by reducing the available import factor KPNB1 (Kβ1). Accordingly, various types of the IFNs are secreted from viral-infected cells that may induce various anti-viral restriction factors (such as OAS, PKR, viperin tetherin, IFITM, RNase L, GTPase, TRIM, ADAR1, APOBEC, and others) following binding to their receptors. Coronavirus-derived nonstructural protein also contributes to abrogate IFN expression or IFN-related signaling pathways. SARS-CoV-derived N protein can inactivate anti-virus restriction factor TRIM25 (RING-finger E3 ubiquitin ligase that controls RIG-I ubiquitination and IFN-β production). MAVS/ISP-1-related pathways may cause apoptosis through the induction of the caspases-mitochondria-mediated pathway.