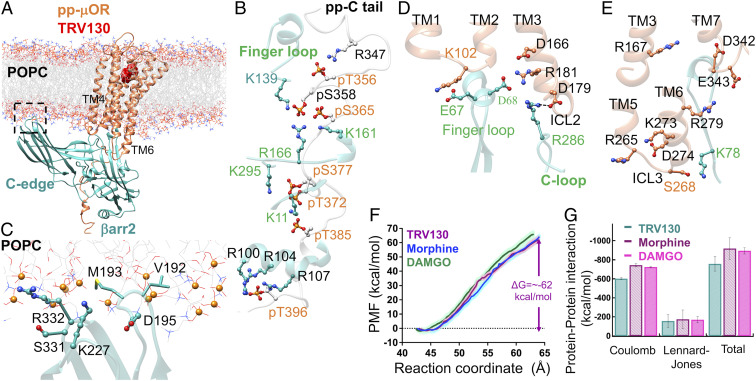
Fig. 6.
(A) The high-affinity βarr2-pp-μOR-TRV130 immersed in the membrane bilayer. (B) Strong interactions between the N domain of βarr2 and the pp-C tail of the μOR, mostly involving pS and pT residues on the pp-C tail with positively charged residues on the N domain of βarr2. (C) The membrane anchoring from the C edge of βarr2, which is mostly dominated by hydrophobic contacts. Here, the P atoms are shown by orange spheres. (D) The polar anchor from βarr2 to ICL2 of μOR, which creates a polar network of interactions from the finger loop to ICL2 and the cytosolic end of TM2. (E) The lack of polar anchoring from βarr2 to ICL3 or the bottom end of TM6 hinders the proper coupling of βarr2 to the core of μOR. (F) The averaged potential of mean force (PMF) for βarr2 coupling to the 7TM core μOR. The free energy was obtained by umbrella sampling where the reaction of coordinate is the distance along the z component between the center of mass of Cαs in the pp-μOR for residues 54 to 340 and the center of mass of Cαs in βarr2. The errors (shaded as pink for TRV130, light green for DAMGO, and light blue for morphine) were assessed by the bootstrap method (38). (G) The nonbonded interactions within 12 Å between βarr2 and the core of pp-μOR for DAMGO (full), morphine (partial), and TRV130 (biased) agonist in the fully engaged complexes, where we excluded water, and ions from these calculations.