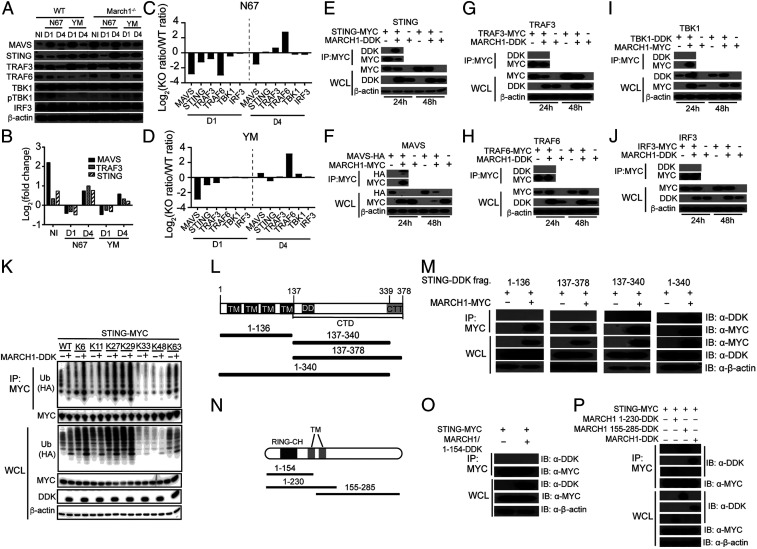
Fig. 3.
MARCH1 interacts with and regulates key molecules in IFN-I signaling pathways. (A) Western blotting analysis of proteins from spleens of uninfected (NI) or day 1 (D1) or day 4 (D4) infected WT and March1−/− mice. Antibodies against host proteins are as indicated (Left). pTBK1 was detected using an anti-phosphorylation antibody. β-Actin was used as protein loading control. (B) Log2(fold change) of protein expression (March1−/− over WT) scanned from Western blot signals in A after adjusting for protein loading. (C and D) Plots of ratios of scanned protein signals from March1−/− mice over those of WT mice in A after adjusting for protein levels from uninfected mice and protein loading. Signals of key proteins in IFN-I response from day 1 and day 4 infected mice were first divided by those of day 0 (uninfected) to adjust for the differences in basal protein levels of the uninfected March1−/− and WT mice. (E–J) Western blots of immunoprecipitated proteins (Top two panels) and total protein expression (Bottom three panels) from 293T cells transfected with plasmids containing molecules as indicated. Anti-MYC, -HA, or -DDK antibodies were used to pull down and detect proteins as indicated. WCL, whole-cell lysate. (K) STING ubiquitination in the presence or absence of MARCH1. 293T cells were transfected with STING-MYC and ubiquitin-HA molecules having a specific available lysine (K) site plus or minus MARCH1-DDK. STING-MYC was pulled down (immunoprecipitated) using anti-MYC antibody. Ubiquitination levels of STING were detected using an anti-HA antibody for HA-tagged ubiquitin. (L) Diagrams of STING deletion constructs, indicating the numbers of amino acids in each construct. (M) Co-IP to detect binding of STING with MARCH1 fragments as shown in L. (N) Diagrams of MARCH1 deletion constructs, indicating the numbers of amino acids in each construct. (O and P) Co-IP to detect binding of STING with MARCH1 fragments as shown in M. IB, immunoblotting.