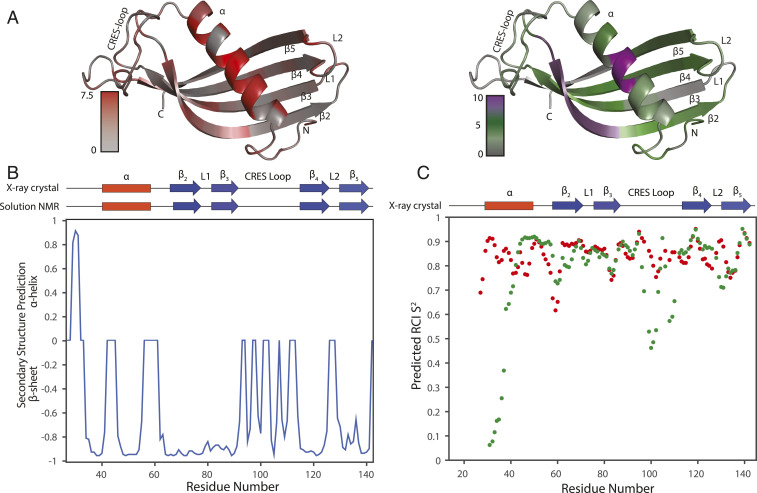
Fig. 5.
Structural analysis of CRES using ssNMR chemical shift assignments. (A) The absolute value of CSPs between solid-state and solution-state NMR mapped onto solution-state NMR structure (shaded from gray to red) and AmylPred2 predicted amyloidogenic sites of the protein (shaded from gray [no prediction] to green [at least one prediction] to purple [unanimous prediction]). (B) Protein secondary structure predicted from ssNMR assigned chemical shifts using TALOS-N. Secondary structure components of CRES X-ray crystal structure and solution NMR are presented in red rectangles for α-helices and blue arrows for β-sheets. (C) Dot plot of predicted RCI S2 from ssNMR (red) and solution NMR (green) chemical shifts.