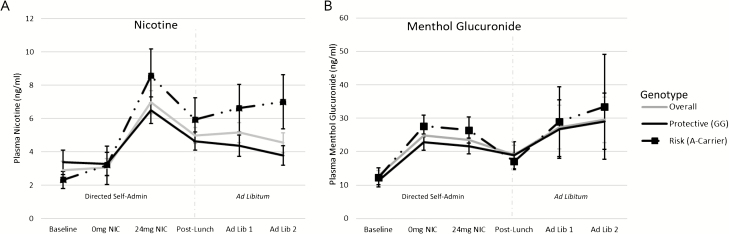
Figure 3.
Validation of Nicotine and Menthol Delivery. (A) Plasma Nicotine and (B) the menthol metabolite Menthol Glucuronide were assessed at six blood draw timepoints. In order, the timepoints were (1) Baseline (following overnight smoking abstinence and prior to e-cigarette self-administration), (2) 0 mg NIC (following 0 mg/mL nicotine Directed Self-Administration Block 1), (3) 24 mg NIC (following 24 mg/mL nicotine Directed Self-Administration Block 2), (4) Post-Lunch (30 min break from e-cigarette administration, including lunch; timepoint also served as a baseline for the Ad Libitum Component), (5 & 6) Ad Lib 1 and 2 (Ad Libitum blocks 1 and 2 [15 min each, separated by 10 min break]). The dotted gray line separates data points included in manipulation-check analyses validating nicotine and menthol delivery during directed self-administration and expected decreases following lunch (timepoints 1–4) and analyses assessing volume of nicotine and menthol self-administration during ad libitum (as a secondary outcome measure; timepoints 4–6). Means (standard error [SE]) are presented for the sample overall (gray) and split by genotype type group (black solid line = ‘Protective (GG)’, black dotted line with square markers = ‘Risk (A-Carrier)’.