Figure 4. Residues critical for FusA/E/B ternary complex.
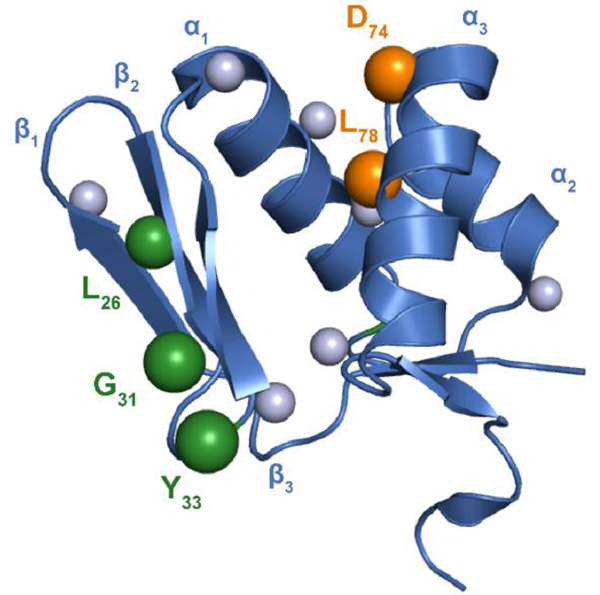
The RRE domain of MccB (residues M1-S86, PDB entry 3H9J, blue) was aligned with FusE. Spheres indicate residues of FusE probed by mutagenesis. Mutagenesis at sites along α3 drastically reduced binding to FusAleader (orange). Ala-substitutions along β2 and β3 did not influence FusAleader binding but did abolish leader peptide cleavage (green). The validity of these predicted protein-protein interaction sites is underscored by the fact mutagenesis elsewhere (gray) did not reduce leader peptide binding or leader peptidase activity.
