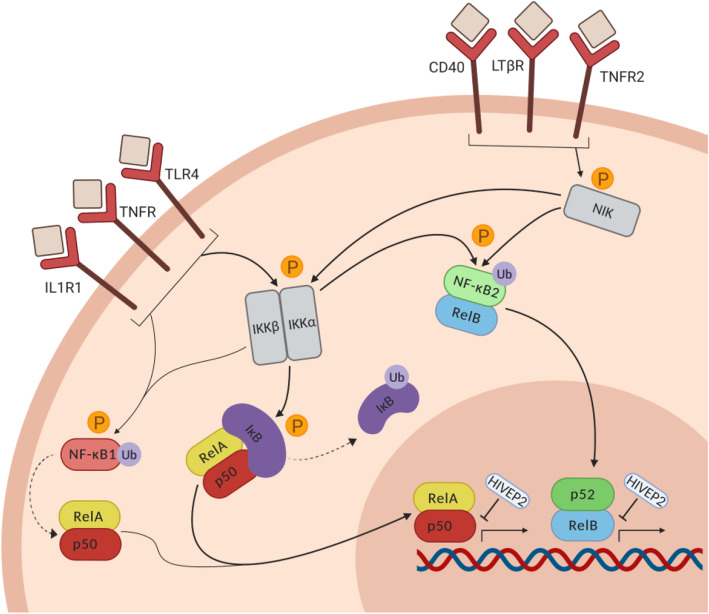
Fig. 1.
The NF-κB activation pathway. Receptors of the canonical arm of the pathway include IL1R1, TNFR, and TLR4. Activation of these receptors leads to the phosphorylation of both IKK and NF-κB1. The activated IKK complex tags IκB for proteasomal degradation, unmasking the nuclear localization sequence on RelA and allowing for nuclear localisation of the p50-RelA heterodimer. Activated IKKβ also phosphorylates NF-κB1, which is partially processed by the proteasome into mature subunit p50. p50 most commonly dimerizes with RelA, and the dimer either translocates to the nucleus or binds IκB and remains in the cytoplasm. Receptors of the non-canonical arm of the pathway include CD40, LTβR, and TNFR2. Activation of these receptors leads to the phosphorylation of NIK, which activates IKKα. Both NIK and IKKα are required for the phosphorylation of NF-κB2, which acts as an inhibitor while bound to RelB in the cytoplasm. Once tagged by NIK/IKKα, NF-κB2 undergoes partial processing by the proteasome into mature subunit p52. p52-RelB dimers then translocate to the nucleus. Rel-containing NF-κB dimers in the nucleus bind target gene DNA in a dimer- and cell type-specific manner to initiate transcription. HIVEP2 is a zinc finger protein that blocks NF-κB-induced gene transcription by binding κB sites on DNA