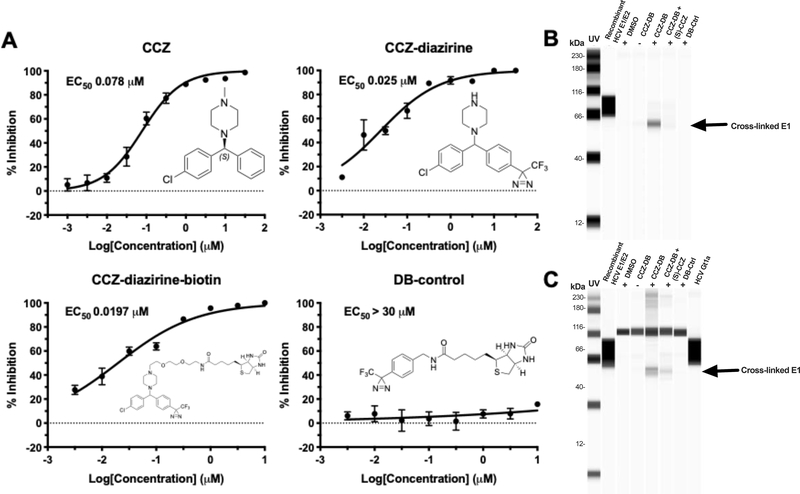
Figure 4. Characterization of CCZ-diazirine derivatives and UV-activated crosslinking of CCZ-DB with HCV E1 protein.
A. Structures and dose-response curves of (S)-CCZ, CCZ-diazirine, CCZ-diazirine-biotin (CCZ-DB) and diazirine-biotin (DB) control. CCZ-diazirine and CCZ-diazirine-biotin were active in inhibiting HCV in the HCV infection assay, with EC50 of 25.0 nM and 19.7 nM, respectively. The DB control has the diazirine-biotin moieties but was not active against HCV. B. Cross-linking of CCZ-DB with recombinant HCV E1/E2 protein. Purified recombinant E1/E2 protein (genotype 1a) was incubated with various compounds described above at room temperature for 1 h, subjected to UV cross-linking and then purified by Neutravidin beads followed by Western blot with anti-E1 antibody. Recombinant E1/E2 protein was included on the blot as a reference. In one sample, a 100-fold higher concentration of (S)-CCZ (100 μM) was added to the CCZ-DB cross-linking condition. C. Cross-linking of CCZ-DB with E1 protein of HCV genotype 1a-infected cells. Huh7.5.1 cells were infected with high-titer HCVcc genotype 1a virus in the presence of the various compounds at 37°C for 1 h, subjected to UV cross-linking and then lysed for purification by Neutravidin beads followed by Western blot with anti-E1 antibody. The same conditions were tested as the HCV recombinant E1/E2 protein above. A high-titer stock of the HCV genotype 1a virus was also run on the blot to demonstrate the presence of E1 protein. The results are representative of three separate experiments.