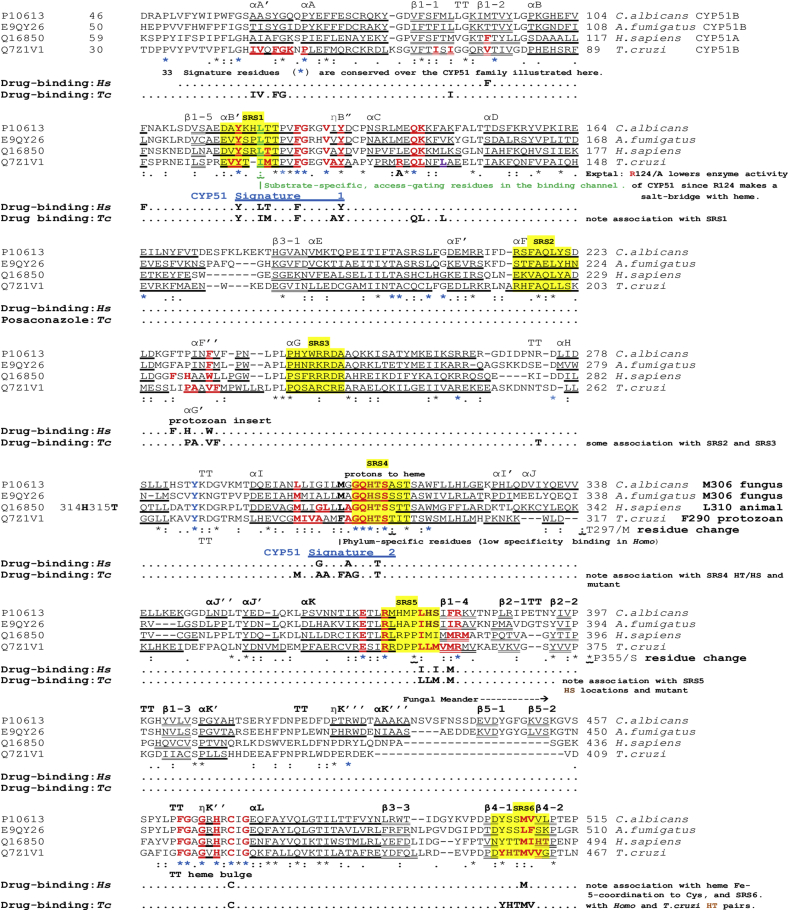
Fig. 5.
Clustal O alignment of sterol 14α-demethylase (CYP51) from parasitic Fungi,Candida albicansandAspergillus fumigatus, in comparison with theHomosapiensand the parasiticT. cruzienzymes. Residues in the CYP51 sterol-binding channel up to 4.5 Å from heme are bold red throughout. Drug-binding sites are noted in association with these residues. The α-helical and 310-helical η(eta)-sequences are boldly underlined, while β-strands are doubly underlined. TT indicates a turn, seen between parallel or antiparallel helices or β-strands, or in other locations, and usually indicates exposure of the turn residues to solvent. Six conserved Substrate Recognition Sites (Gotoh, 1992; Lepesheva and Waterman, 2011; Strushkevich et al., 2010) are located with a yellow background, arbitrarily including 8 residues and include SRS 4 with its His/Thr scouple in α-helix I, facilitating the delivery of protons (H+) to oxygenated heme in the substrate pocket. Histidine here is a conserved signature residue (*) which is followed by Thr or Ser, and immediately followed by the remainder of helix I. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)