Fig. 6.
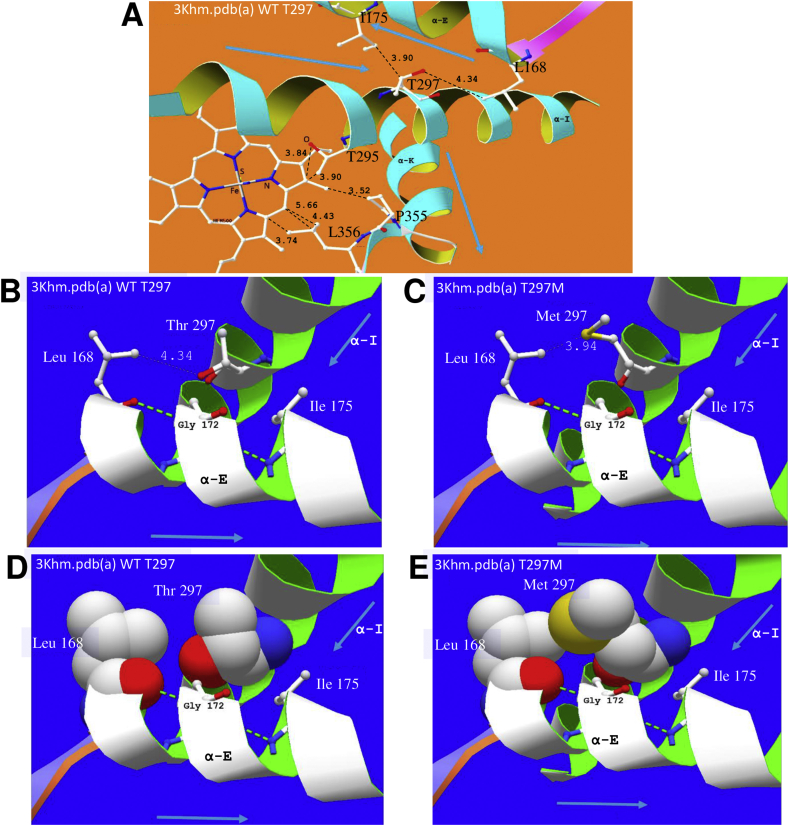
Impact of residue changes T297M and P355S on the active site of CYP51. A (3khm.pdb (a) WT) The area around active site heme showing helix E and its VdW hydrophobic contact with signature helix I, through the side-chains of, for example, L168, I175 and T297/M297. This also shows helix K crossing helix I and supporting the following β-sheet bearing P355/S355 and L356, which support the heme. Helix I also supports heme through T295, among other residues. The residue change P355S replaces a highly conserved turn-defining residue in VdW hydrophobic contact with the heme and is next to residue L356, which also contacts heme. As can be seen from our alignment (Fig. 5) L356 binds all 7 drugs looked at in crystals of the T. cruzi - T. brucei enzyme. B and C show VdW distance change associated with T297M. D and E show the increased size of the sidechain of M297, four times more hydrophobic and 90 Å larger than that of T297, thus likely to reduce by 15% the solvent-accessible volume at the enzyme active site, estimated by Lepesheva and collaborators (Lepesheva et al., 2010) as 600 Å3 in T. brucei. This may hinder the access of drug to coordination at the 6th site of the heme iron as well as having some influence on access of the sterol substrate to the enzyme.