Abstract
Background
Programmed death-ligand 1 (PD-L1) is a negative costimulatory molecule, and its main function is widely considered to be in the regulation of T cells. Tumor-associated macrophages (TAMs) are an important part of the tumor microenvironment, and they also play an important role in immunosuppression. However, the relationship between the expression of PD-L1 and TAMs in cervical carcinoma (CC) remains unclear. We detected the expression of PD-L1 and TAMs in tumor tissue to study the correlation between them.
Methods
Immunohistochemical staining of PD-L1, CD68 (pan-macrophage), and CD163 (M2-like macrophage) was performed in 120 cases of cervical squamous cell carcinoma. Logistic regression analysis was used to evaluate the predictors related to positive PD-L1 expression. We also apply the Kaplan–Meier method to study the recurrence-free and overall survival rate of CC patients.
Results
The increase in PD-L1 expression in tumor cells (TC) was significantly correlated with the increase in CD163 density (r=0.8550, p<0.0001), while PD-L1 in the stroma was also significantly associated with the intratumoral density of CD68- or CD163-positive cells (CD68 p<0.0001; CD163 p=0.0009). The mean infiltration rates of CD68- and CD163-positive cells in PD-L1-positive TC were significantly higher than in PD-L1-negative TC (CD68 p=0.0095; CD163 p<0.0001). In multivariate logistic regression analyses, only the density of CD163-positive cells was correlated with the expression of PD-L1 in TC cells (OR 1.52; p=0.032). In prognostic analysis, PD-L1 more than 10% was significantly correlated with short RFS (HR=2.66; p=0.028). For CD163+ macrophage evaluation, the density above the median was also significantly correlated with RFS (HR=2.48; p=0.021).
Conclusion
CD163+ M2-like macrophage infiltration is highly associated with PD-L1 expression in CC, suggesting that macrophage infiltration can serve as a potential therapeutic target.
Keywords: cervical cancer, programmed death-ligand 1, macrophages, tumor microenvironment
Introduction
Despite efforts to improve the prevention and treatment of cervical cancer, cervical cancer (CC) is the fourth most common cause of death from cancer among women in the world.1 According to the 2018 Global Cancer Statistics report,2 the incidence and mortality of CC account for 6.6% and 7.5% of all female cancer patients, respectively. In China, the age of cervical cancer onset is decreasing, and Xinjiang has a high incidence of cervical cancer.3 According to statistics, the median overall survival rate of advanced cervical cancer was only 16.8 months.4 Although several prognostic biomarkers have been identified, such as lymph node status, tumor size, stage, and pathological differentiation, these biomarkers lack specificity and sensitivity for accurate prediction. Therefore, it is urgent to identify many novel and feasible prognostic markers to guide individualized treatment and predict the survival outcomes of patients with cervical cancer.
Programmed death-ligand 1 (PD-L1, also termed CD274 or B7-H1) is the main ligand of programmed cell death protein 1 (PD-1). PD-L1 is expressed in immune cells, including activated T lymphocytes, B lymphocytes, dendritic cells, macrophages, and many tumor cells.5 In general, PD-L1 expression maintains the homeostasis of the immune response. In a healthy immune system, the activation of the PD-1/PD-L1 pathway can limit autoimmunity and inhibit the activity of T lymphocytes under the inflammatory response of infection.6 In the tumor microenvironment (TME), cancer cells and infiltrating immune cells express PD-L1, which binds to PD-1 on T lymphocytes and then inhibits the proliferation and effect of T cells.7,8
Because PD-1 and its ligands inhibit the response of T cells to tumor cells, PD-1/L1 inhibitors have been developed for clinical application and proved to be effective for several cancers.9–11 In June 2018, FDA approved pembrolizumab for the treatment of recurrent or advanced cervical cancer patients with PD-L1 positive expression (KEYNOTE-158 clinical registration trial).12 In patients with cervical cancer, discovering the mechanisms and predictive markers of PD-L1 expression can help clinicians further refine the selection of patients who are most likely to benefit from PD-1/L1 inhibitors. However, in the tumor microenvironment, especially the infiltration of immune cells, it seems to be related to the expression of PD-L1. Tumor-associated macrophages (TAMs) comprise the largest number of inflammatory immune cells in TME, accounting for about 30% of them. At present, the role of PD-1/PD-L1 inhibitor interaction in regulating the inhibition of T lymphocytes has been gradually established, but the expression and effect of PD-L1 on macrophages need to be further studied.
Existing studies have found that the density of macrophages expressing PD-L1 in tumors can predict the efficacy of PD-1/L1 inhibitors.13–15 In some cases, a high response rate of up to 80% was observed in at least 10% of patients whose TAMs expressed PD-L1.13 In the screening of several different human tumors, macrophages expressing PD-L1 are more abundant than tumor cells.13 Moreover, anti-PD-L1 therapy can induce antitumor activity even in the model in which tumor cells do not express PD-L1, which suggests that the expression of PD-L1 in macrophages may be a key factor in promoting the therapeutic response to the PD-L1 antibody.14,15 In the current study, we stained PD-L1, CD68 (pan-macrophage) and CD163 (M2-like macrophage) in 120 CC specimens, and evaluated the relationship between PD-L1 expression in CC cells and macrophage infiltration in TME as well as the clinicopathological features.
Materials and Methods
Study Cohort
This study retrospectively evaluated 120 tissue samples of squamous cell carcinomas from patients who underwent surgery in the Third Clinical Medical College of Xinjiang Medical University (Affiliated Tumor Hospital, Urumqi, Xinjiang) from January 2012 to December 2014. Along with the tumor paraffin specimens, clinical characteristics were also collected. Patients who received chemotherapy or radiotherapy prior to surgery were excluded. Each paraffin specimen included 5 pathological tissue sections. All cases were confirmed by surgery and pathology. The staging of cervical cancer was made according to the International Federation of Gynecology and Obstetrics (FIGO) recommendations in 2018.16 The median follow-up time of the censored cases was 5.92 years (range, 5.08–8 years) from the date of surgery. The prognosis of recurrence-free survival (RFS) and overall survival (OS) was analyzed. RFS was defined as an interval between the time of initial diagnosis and recurrence, last follow-up, or death. OS was defined as an interval from the initial diagnosis to death or last follow-up. All procedures were followed according to the ethical standards of institutions and national committees on human experimentation and were consistent with the Helsinki Declaration of 1964 and later versions. The study and consent procedure was approved by the Ethics Committee of the third Clinical Medical College of Xinjiang Medical University (affiliated Tumor Hospital). Informed consent or an alternative was obtained from all patients included in the study.
Immunohistochemistry
Formalin-fixed paraffin-embedded surgical specimens were sectioned at 2 mm and mounted on glass slides. The slices were dried for 1.5 h at 60°C, then these sections were dewaxed in xylene and graded alcohols, hydrated and washed in phosphate-buffered saline (PBS). After placing in a microwave oven for pretreatment (PD-L1: EDTA antigen repair solution for 30 min, pH 9; CD68 and CD163: sodium citrate buffer for 15 min, pH 6), endogenous peroxidase was inhibited by 3% H2O2 for 30 min, then the sections were incubated with 10% normal goat serum for 40 min. Primary antibodies composed of rabbit anti-PD-L1 antibody 28–8 (diluted 1:100, ab205921, Abcam, Cambridge, UK), rabbi anti-CD68 antibody (diluted 1:50, BA3638, Boster, California, UK), or rabbit anti-CD163 antibody (diluted 1:200, bs-2527R, bioss, Beijing, China), were applied overnight in a moist chamber at 4°C. The next day, the tissues were incubated with secondary antibodies, stained with diaminobenzidine (DAB), and counterstained with hematoxylin. Immunohistochemistry was manual staining. Phosphate-buffered saline, rather than the primary antibody, was the negative control in the IHC; the previous positive specimen was the positive control.
Immunohistochemical Evaluation
PD-L1 expression in tumor cells and stroma was immunohistochemically analyzed by two independent pathologists without knowledge of clinical data or CD68- or CD163-positive cell density. We calculated the proportion of positive cells (10% or less was negative and more than 10% was positive) by selecting the nests of cervical cancer including more than 100 cancer cells. The positive criterion is in the cytomembrane and cytoplasm or both at the same time appeared tan particles. Two patients’ samples could not be evaluated for PD-L1 expression owing to the sample condition.
Macrophage infiltration was quantitatively estimated in the stroma of tumor field. The positive expression of CD68 and CD163 antibody was dark brown in the cytoplasm. Each sample was screened at a low magnification (× 100), and five regions with the maximum number of positive staining cells (hot spots) were selected for further analysis. The average macrophage count (per mm2) in these five regions of each case was estimated at a high power (× 400) magnification rate. The density of CD68- and CD163-positive cells was evaluated by computer-aided image analysis and Image J software. The density was calculated as the positive staining areas divided by the total analytical areas. For the five ‘hot spots’, the averages were used to represent the final macrophage density (MD) on each slide.
The average MD was determined by two independent pathologists who had no knowledge of the patient’s pathological and clinical status. In order to confirm the reproducibility, 25% glass tablets were randomly selected and scored twice. And we evaluated all the results in a similar way. Due to the sample conditions, it was not possible to evaluate the CD163-positive cell region of five patients’ samples.
Statistical Methods
IBM SPSS Statistics Software, version 26 and GraphPad Prism 8 were used for all statistical analyses. All p-values were two-sided, and below 0.05 were considered statistically significant. Independent-samples t-test or ANOVA test was used, and the Kruskal–Wallis test was used with unequal variances. Chi-square test was used to compare the sample rates. Pearson’s chi-square test or Fisher’s exact test was used to assess the association between categorical variables, accordingly. We performed univariate and multivariate logistic regression analyses to assess the predictors associated with positive PD-L1 expression in TC cells and estimated the odds ratios with 95% confidence intervals. Factors with p < 0.15 in the univariate analyses were entered into the multivariate analyses. Finally, the Cox regression model was used to assess the hazard ratio (HR) of mortality. For macrophages, patients were grouped according to the median of infiltrating CD68- and CD163-positive cells. The cumulative recurrence-free survival rate was calculated by the Kaplan–Meier method and analyzed by the Log-rank test.
Results
PD‑L1 Expression and CD68+ and CD163+ Macrophage Infiltration in CC
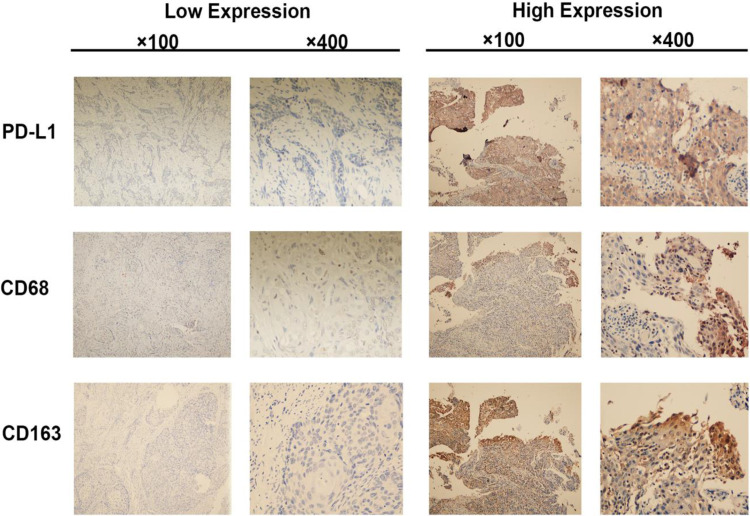
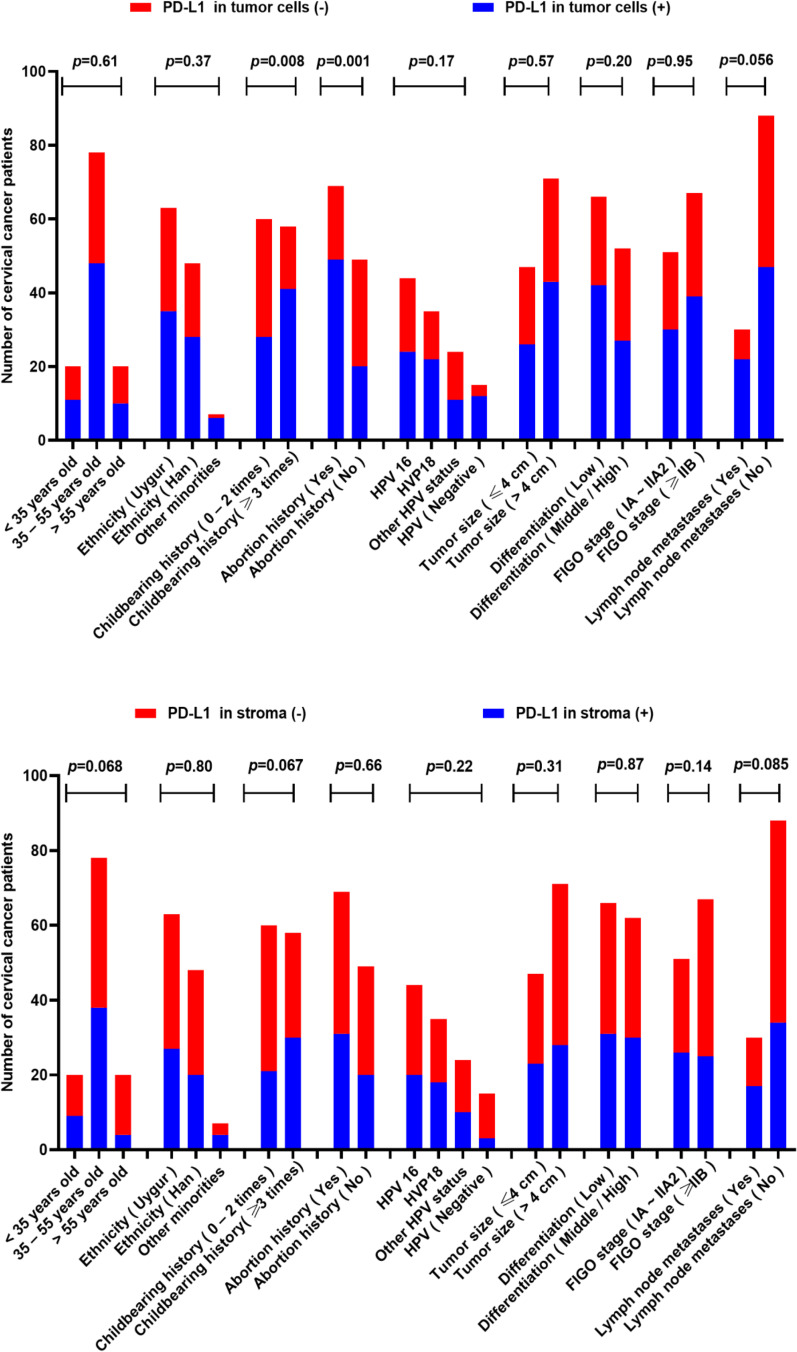
Figure 1 shows the IHC staining images of PD-L1, CD68, and CD163. Of the 118 evaluable CC samples, 69 (58.47%) stained positive for PD-L1 expression in cancer cells, and 51 (43.22%) stained positive in the stroma (Table 1). The PD-L1 expression was greater in the groups with ≥3 fertility history and abortion history (both p<0.05) (Figure 2). The expression of PD-L1 in TC was significantly different in the childbearing history and abortion history of the patients (both p<0.05) (Figure 2).The intratumoral density of CD68-positive cells in 120 CC samples was distributed as follows: mean 13.84; median 14.2; standard deviation 5.83; range 2.8–29; interquartile range 10.5–18.8 (Table 2). The distribution of intratumoral density of CD163-positive cells in 115 evaluable CC samples was as follows: mean 13.33; median 12.6; standard deviation 7.30; range 2.5–28.7; interquartile range 6.7–19.2 (Table 2). The intratumoral density of CD163-positive cells in the FIGO stage (≥IIB group) was significantly higher than that in the IA ~ IIA2 group (p<0.05; Table 2). The intratumoral density of CD163-positive cells in cervical carcinoma with lymph node metastasis was significantly higher than that in the non-lymph node metastasis group (p<0.05; Table 2). However, the intratumoral density of CD68-positive cells was not associated with the FIGO stage and lymph node metastasis.
Figure 1.
Immunohistochemistry staining for PD-L1, CD68, and CD163 expression in cervical squamous cell carcinoma tissues. The left panel shows negative staining for PD-L1 and low macrophage density. The right panel shows positive staining for PD-L1 and high macrophage density.
Table 1.
PD-L1 Expression (via Immunohistochemistry) and Clinical Characteristics of 118 Patients with Cervical Squamous Cell Carcinoma
| Clinical or Pathologic Features | Total No. | PD-L1 Expression in Tumor Cells | PD-L1 Expression in Stroma | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | p value | Positive | Negative | p value | ||
| All cases | 118a | 69(58.5) | 49(41.5) | - | 51(43.2) | 67(56.8) | - |
| Age at diagnosis | 0.61 | 0.068 | |||||
| < 35 years old | 20 | 11(55.0) | 9(45.0) | 9(45.0) | 11(55.0) | ||
| 35–55 years old | 78 | 48(61.5) | 30(38.5) | 38(48.7) | 40(51.3) | ||
| >55 years old | 20 | 10(50.0) | 10(50.0) | 4(20.0) | 16(80.0) | ||
| Ethnicity | 0.37 | 0.80 | |||||
| Uygur | 63 | 35(55.6) | 28(44.4) | 27(42.9) | 36(57.1) | ||
| Han | 48 | 28(58.3) | 20(41.7) | 20(41.7) | 28(58.3) | ||
| Other minorities | 7 | 6(85.7) | 1(14.3) | 4(57.1) | 3(42.9) | ||
| Childbearing history | 0.008 | 0.067 | |||||
| 0–2 times | 60 | 28(46.7) | 32(53.3) | 21(35.0) | 39(65.0) | ||
| ≥ 3 times | 58 | 41(70.7) | 17(29.3) | 30(51.7) | 28(41.3) | ||
| Abortion history | 0.001 | 0.66 | |||||
| Yes | 69 | 49(71.0) | 20(29.0) | 31(44.9) | 38(55.1) | ||
| No | 49 | 20(40.8) | 29(59.2) | 20(40.8) | 29(59.2) | ||
| HPV status | 0.17 | 0.22 | |||||
| HPV 16 | 44 | 24(54.5) | 20(45.4) | 20(45.5) | 24(54.5) | ||
| HVP18 | 35 | 22(62.9) | 13(37.1) | 18(51.4) | 17(48.6) | ||
| Other | 24 | 11(45.8) | 13(54.2) | 10(41.7) | 14(58.3) | ||
| Negative | 15 | 12(80.0) | 3(20.0) | 3(20.0) | 12(80.0) | ||
| Tumor size | 0.57 | 0.31 | |||||
| ≤ 4 cm | 47 | 26(55.3) | 21(44.7) | 23(48.9) | 24(51.1) | ||
| > 4 cm | 71 | 43(60.6) | 28(39.4) | 28(39.4) | 43(60.6) | ||
| Differentiation | 0.20 | 0.87 | |||||
| Low | 66 | 42(63.6) | 24(36.4) | 31(47.0) | 35(53.0) | ||
| Middle/High | 52 | 27(51.9) | 25(48.1) | 30(57.7) | 32(61.5) | ||
| FIGO stage | 0.95 | 0.14 | |||||
| IA ~ IIA2 | 51 | 30(58.8) | 21(41.2) | 26(51.0) | 25(49.0) | ||
| ≥ IIB | 67 | 39(58.2) | 28(41.8) | 25(37.3) | 42(62.7) | ||
| Lymph node metastases | 0.056 | 0.085 | |||||
| Yes | 30 | 22(73.3) | 8(26.7) | 17(56.7) | 13(43.3) | ||
| No | 88 | 47(53.4) | 41(46.6) | 34(38.6) | 54(61.4) | ||
Notes: Data were shown as number of cases (%). aTwo samples could not be evaluated for PD-L1 owing to sample conditions.
Figure 2.
Clinical significance of PD-L1expression in 118 patients.
Table 2.
Density of CD68- and CD163-Positive Cells in the Tumor Field and Clinical Features in 120 Samples of Cervical Squamous Cell Carcinoma
| Clinical or Pathologic Features | CD68 | CD 163 | ||||
|---|---|---|---|---|---|---|
| Total No. | CD68 (%) (Mean ± SE) |
p value | Total No. | CD163 (%) (Mean ± SE) |
p value | |
| All cases | 120 | 13.84±5.83 | – | 115a | 13.33±7.30 | – |
| Age at diagnosis | 0.54 | 0.64 | ||||
| <35 years old | 20 | 12.98±6.24 | 19 | 12.65±7.85 | ||
| 35–55 years old | 78 | 14.54±7.21 | 77 | 13.45±7.23 | ||
| >55 years old | 22 | 14.03±5.65 | 19 | 13.91±8.21 | ||
| Ethnicity | 0.43 | 0.44 | ||||
| Uygur | 64 | 15.33±5.98 | 60 | 14.21±8.54 | ||
| Han | 49 | 13.96±6.18 | 48 | 13.29±8.02 | ||
| Other minorities | 7 | 12.23±7.23 | 7 | 12.51±9.21 | ||
| Childbearing history | 0.12 | 0.24 | ||||
| 0–2 times | 62 | 14.21±6.21 | 57 | 12.79±8.32 | ||
| ≥ 3 times | 58 | 13.47±6.89 | 58 | 13.87±8.82 | ||
| Abortion history | 0.46 | 0.09 | ||||
| Yes | 71 | 13.01±6.32 | 69 | 14.02±8.76 | ||
| No | 49 | 14.67±5.99 | 46 | 12.64±9.13 | ||
| HPV status | 0.37 | 0.32 | ||||
| HPV 16 | 45 | 14.88±5.91 | 44 | 14.65±7.98. | ||
| HVP18 | 36 | 14.84±6.27 | 35 | 14.34±8.32 | ||
| Other | 24 | 13.12±6.93 | 23 | 13.01±8.54 | ||
| Negative | 15 | 12.54±7.32 | 13 | 11.32±10.21 | ||
| Tumor size | 0.52 | 0.84 | ||||
| ≤ 4 cm | 47 | 13.21±6.98 | 44 | 13.35±9.21 | ||
| > 4 cm | 72 | 14.45±6.12 | 71 | 13.31±8.35 | ||
| Differentiation | 0.18 | 0.21 | ||||
| Low | 68 | 13.45±6.01 | 66 | 13.99±8.02 | ||
| Middle/High | 52 | 14.23±6.92 | 49 | 12.67±9.15 | ||
| FIGO stage | 0.12 | 0.04 | ||||
| IA ~ IIA2 | 51 | 13.03±7.21 | 50 | 12.03±8.87 | ||
| ≥ IIB | 69 | 14.65±6.34 | 65 | 14.63±8.12 | ||
| Lymph node metastases | 0.06 | 0.03 | ||||
| Yes | 32 | 14.88±8.32 | 30 | 15.23±9.21 | ||
| No | 88 | 12.81±6.31 | 85 | 11.43±8.13 | ||
Note: aFive samples could not be evaluated for CD163 owing to sample conditions.
Abbreviation: SE, standard error.
Associations Between PD-L1 Expression and CD68- or CD163-Positive Cell Intratumoral Density in CC
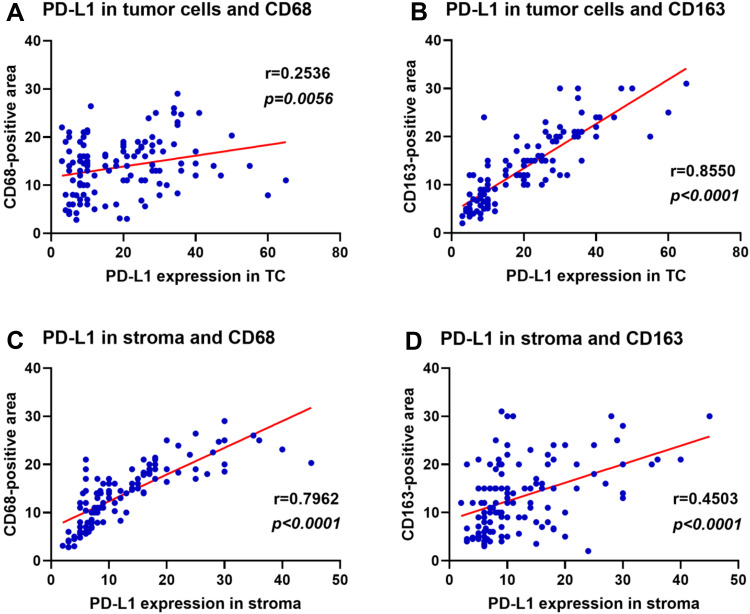
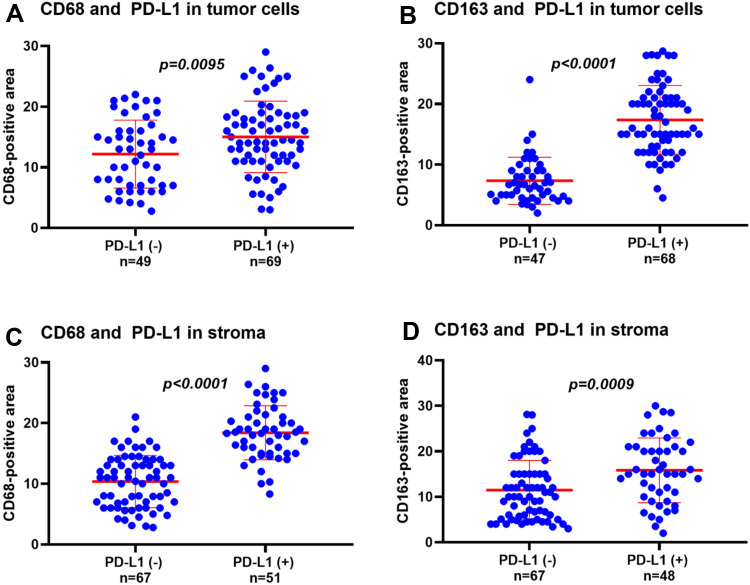
We examined the correlation between the expression of PD-L1 and the intratumoral density of CD68- and CD163-positive cells in cervical carcinoma (Figure 3). The increase of PD-L1 expression in TC was significantly correlated with the increase of CD163 density (r=0.8550, p<0.0001), but weakly correlated with the increase of CD68 density (r=0.2536, p=0.0056). Conversely, the increase of PD-L1 expression in the stroma was strongly correlated with the increase of CD68 density (r=0.7962, p<0.0001), but moderately correlated with the increase of CD163 density (r=0.4503, p<0.0001). We then examined the relationship between the density of CD68- and CD163-positive cells and the expression of PD-L1 in cervical carcinoma (Figure 4). The density of CD68- and CD163-positive cells in the PD-L1 positive group in TC was significantly higher than that in the PD-L1 negative group (CD68 p=0.0095; CD163 p<0.0001). These results indicated that the correlation between CD163 density and PD-L1 expression in TC was stronger than that of CD68 density. The expression of PD-L1 in the stroma was also significantly associated with the intratumoral density of CD68- or CD163-positive cells (CD68 p<0.0001; CD163 p=0.0009).
Figure 3.
Correlation of PD-L1 expression and density of CD68-and CD163-positive cells in cervical cancer.
Notes: (A) PD-L1 expression in tumor cells and density ofCD68. (B) PD-L1 expression in tumor cells and density of CD163. (C) PD-L1 expression in the stroma and density of CD68. (D) PD-L1 expression in the stroma and density of CD163.
Figure 4.
Relationship between the density of CD68- and CD163-positive cells and the expression of PD-L1 in cervical cancer. (A) Density of CD68 in PD-L1 positive and negative expression groups in TC. (B) Density of CD163 in PD-L1 positive and negative expression groups in TC. (C) Density of CD68 in PD-L1 positive and negative expression groups in stroma. (D) Density of CD163 in PD-L1 positive and negative expression groups in stroma.
Predictive Factors for PD-L1 Expression in TC
In order to identify factors predicting positive PD-L1 expression in tumor cells, univariable and multivariable logistic regression analysis was performed. In univariate analysis, PD-L1 expression in TC was associated with childbearing history, abortion history, and CD163-positive cell density (Table 3). In the multivariate analysis, which included features with p < 0.1 in the univariate analysis (childbearing history, abortion history, FIGO stage, and CD163-positive cell density), only the density of CD163-positive cells was correlated with PD-L1 expression in TC (odds ratio 1.52; 95% confidence interval 1.31–1.89; p = 0.032; Table 3).
Table 3.
Univariate and Multivariate Analysis of Factors Associated with PD-L1 Expression in Tumor Cells
| Clinical or Pathologic Features | Univariate | Multivariate | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p value | Odds Ratio (95% CI) | p value | |
| Age at diagnosis | ||||
| < 59 years old | 1 (reference) | |||
| ≥50 years old | 1.66(0.78–3.54) | 0.19 | ||
| Ethnicity | ||||
| Uygur | 1 (reference) | 0.49 | ||
| Han | 0.38(0.07–2.06) | |||
| Other minorities | 0.48(0.09–2.72) | |||
| Childbearing history | ||||
| 0–2 times | 1 (reference) | 1 (reference) | ||
| ≥ 3 times | 2.70(1.24–5.88) | 0.012 | 0.08(0.01–1.04) | 0.053 |
| Abortion history | ||||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 2.59(1.20–5.58) | 0.015 | 0.07(0.01–0.94) | 0.44 |
| HPV status | ||||
| HPV 16 | 1 (reference) | 0.15 | ||
| HVP18 | 0.27(0.07–1.11) | |||
| Other | 0.41(0.10–1.70) | |||
| Negative | 0.20(0.05–0.86) | |||
| Tumor size | ||||
| ≤ 4 cm | 1 (reference) | |||
| > 4 cm | 2.89(0.48–17.49) | 0.25 | ||
| Differentiation | ||||
| Middle/High | 1 (reference) | |||
| Low | 2.35(0.63–8.71) | 0.20 | ||
| FIGO stage | ||||
| IA ~ IIA2 | 1 (reference) | 1 (reference) | ||
| ≥ IIB | 0.20(0.03–1.23) | 0.082 | 1.70(0.33–8.83) | 0.53 |
| Lymph node metastases | ||||
| No | 1 (reference) | |||
| Yes | 1.43(0.57–3.59) | 0.44 | ||
| CD68-positive cell densitya | 0.94(0.82–1.07) | 0.31 | ||
| CD163-positive cell densitya | 1.61(1.35–1.93) | 0.0001 | 1.52(1.31–1.89) | 0.032 |
Note: aContinuous variable.
Abbreviation: CI, confidence interval.
Prognosis Analysis
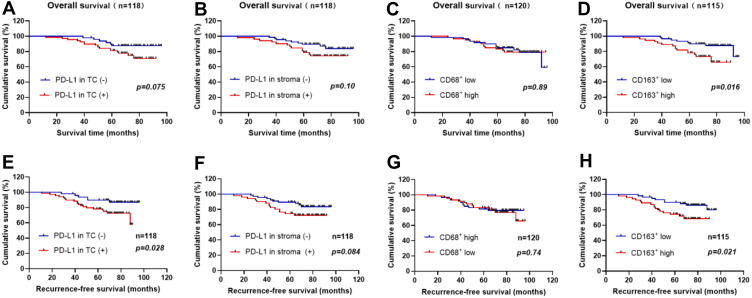
Among the 120 evaluated patients, 23 patients had died at the last follow-up period. The median follow-up time for censored patients was 5.92 years. We performed a Cox regression analysis of overall and recurrence-free survival. As we can see from Figure 5 and Table 4, for PD-L1 expression in tumor cells, PD-L1 more than 10% was significantly associated with short RFS (HR = 2.66; p = 0.028; Figure 5E). However, PD-L1 more than 10% was not significantly associated with OS (HR = 2.26; p = 0.075; Figure 5A). Similarly, PD-L1 expression was not significantly associated with OS (HR = 2.0; p = 0.10; Figure 5B) and RFS (HR = 2.02; p = 0.084; Figure 5F) in stroma. For CD163+ macrophage assessments, a density above median in stroma was significantly associated with short OS (HR = 2.68; p = 0.016; Figure 5D) and RFS (HR = 2.48; p = 0.021; Figure 5H). The density of CD68+ macrophages in stroma was not significantly associated with OS (HR = 1.06; p = 0.89; Figure 5C) and RFS (HR = 0.88; p = 0.74; Figure 5G).
Figure 5.
Recurrence-free and overall survival curves in CC patients. (A) Kaplan–Meier OS curves according to PD-L1 expression in tumor cells (B) Kaplan–Meier OS curves according to PD-L1 expression in stroma (C) Kaplan–Meier OS curves according to the density of CD68-positive cells (D) Kaplan–Meier OS curves according to the density of CD163-positive cells (E) Kaplan–Meier RFS curves according to PD-L1 expression in tumor cells (F) Kaplan–Meier RFS curves according to PD-L1 expression in stroma (G) Kaplan–Meier RFS curves according to the density of CD68-positive cells (H) Kaplan–Meier RFS curves according to the density of CD163-positive cells. (The density of CD68/CD163 above median = high CD68/CD163; The density of CD68/CD163 below median = low CD68/CD163; p-values obtained from Log-rank tests.).
Table 4.
Univariate Analysis of Overall and Recurrence-Free Survival
| Clinical or Pathologic Features | Overall Survival | Recurrence-Free Survival | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p value | Hazard Ratio (95% CI) | p value | |
| PD-L1 expression (tumor cells) | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 2.26 (0.99–5.15) | 0.075 | 2.66 (1.21–5.85) | 0.028 |
| PD-L1 expression (stroma) | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 2.0 (0.86–4.67) | 0.10 | 2.02 (0.89–4.55) | 0.084 |
| CD68-positive cell density | ||||
| Low | 1 (reference) | 1 (reference) | ||
| High | 1.06 (0.47–2.35) | 0.89 | 0.88 (0.41–1.89) | 0.74 |
| CD163-positive cell density | ||||
| Low | 1 (reference) | 1 (reference) | ||
| High | 2.68 (1.17–6.15) | 0.016 | 2.48 (1.12–5.50) | 0.021 |
Abbreviation: CI, confidence interval.
Discussion
In 120 CC specimens, we found that the expression of PD-L1 in TC was strongly associated with the infiltration of CD163-positive cells, while PD-L1 expression in the stroma was correlated with CD68-positive cell infiltration, indicating that macrophage infiltration in TME was related to PD-L1 expression, and M2-like macrophages potentially promoted the expression of PD-L1 in CC tumor cells. The results also suggested that M2 macrophage infiltration could serve as a predictive marker for the expression of PD-L1 in cervical cancer, and M2 macrophages could be applied to a potential therapeutic target. Some previous reports have shown that PD-L1 expression on the surface of tumor cells in patients with CC can range from 30% to 81.3%; in our study, it was 58.5%.17,18 Considering these variations in the literature, we think that the positive rate of PD-L1 in our study is valid. The rate may vary among studies for a number of reasons, including heterogeneity and phenotypic differences in samples, patient differences in fertility or abortion history, differences in cancer stages, differences in antibody sources, and the use of different detection platforms.
For the expression of PD-L1 in TC, our results showed that there were great differences in the childbearing history and abortion history of the CC patients (p = 0.008; p = 0.001). The higher the incidences of fertility and abortion, the higher the expression level of PD-L1. It has been reported that the PD-1/PD-L1 pathway was closely related to the history of pregnancy and delivery.19 Pregnancy could change the immune status of women, especially the cervical environment. Negative immune regulation pathways including the PD-1/PD-L1 pathway could be activated during pregnancy. Cervical cancer is a common oncology of the uterus, and high incidences of fertility and abortion were also risk factors for CC. We speculated that the PD-1/PD-L1 negative immune pathway was closely related to the occurrence of CC, and more incidences of childbearing and abortion might lead to the overexpression of PD-L1.
Many studies have shown that high numbers of TAMs promote tumor growth, disease progression, and poor prognosis.20–22 However, the role of TAMs varied among different solid tumors in a number of studies conducted with human samples. A high density of tumor-associated macrophages was associated with a poor survival rate of gastric cancer, but correlated with better OS in patients with colorectal cancer.23 Moreover, there were contradictory data on the study of TAMs and its micro-distribution in patients with lung cancer.24–26 At present, there are several functional markers of TAMs. The existence of CD163 and CD68 is the key difference factor between different TAMs. The molecular marker of pan-macrophage is CD68, while M2-like macrophage is CD163 and CD23.27 Studies have shown that the development from precancerous cervical lesions to cancer was associated with a large number of infiltrated CD68 macrophages.28,29 Our study found that the intratumoral density of CD68-positive cells was not related to clinical features (including FIGO stage and lymph node metastasis), but the density of CD163-positive cells was associated with FIGO stage and lymph node metastasis. They were significantly increased in the FIGO stage (≥IIB) and lymph node metastasis group (p < 0.05). Studies have shown that M2-like TAMs promoted tumor growth, invasion, and metastasis by secreting growth factors, inhibiting the immune response.21 Our results also confirmed the close relationship between them in CC. At present, several drugs that inhibit TAM invasion and polarization into the M2 tumor phenotype have shown an antitumor effect.22,30 Therefore, the key regulator of alternative activation targeting TAMs will likely become a potential immunotherapy strategy for CC.
We also found that a high density of CD163+ macrophages in the stroma was significantly correlated with short OS and RFS in the prognostic analysis of CC. However, the density of CD68 was not significantly correlated with OS and RFS. Nedergaard et al31 have explored the density of less specific CD68 (pan-macrophage markers) in CC patients and found no significant correlation with tumor recurrence. This supports our results in prognostic analysis. In subsequent studies, the high expression level of PD-L1 in tumor cells was correlated with short RFS (HR = 2.66; p = 0.028; Figure 5E). This also indicated that the expression of PD-L1 in tumor cells is closely related to high-density CD163+ macrophages.
The relationship between TAMs and PD-L1 expression in tumor cells is not clear. Tumor-associated macrophages, the main cells in the tumor stroma, are important components of the inhibitory tumor microenvironment. TAMs domesticated by TME can highly express negative co-stimulation such as PD-L1 and B7-H4 that inhibit T cell response and induce apoptosis of cytotoxicity T cells, thus playing an important role in tumor immune escape.32 With the deepening of the study, A previous report33 showed that the expression of PD-L1 protein in HCC tissues was significantly correlated with tumor-associated macrophage infiltration. Macrophages in vitro could up-regulate the expression of PD-L1 in HCC cell lines. However, Kuang et al34 found that tumor-infiltrating macrophages (TIMs) in hepatocellular carcinoma themselves highly expressed PD-L1 protein, which was related to tumor progress and prognosis. A recent study has shown that TAMs express PD-L1, which was involved in immune-suppressive TME.35 These studies further proved that TAMs with high expression of PD-L1 inhibit the function of tumor-specific T cells, thus blocking PD-L1 can reverse the inhibitory effect of tumor-infiltrating macrophages on T cells. Shima et al36 suggested that TIM was an external regulator of tumor PD-L1 expression in lung adenocarcinoma, which indicated that the combined treatment of targeted tumor PD-L1 and matrix TAMs might be an effective strategy for the treatment of lung cancer. Our study showed that the density of TAMs in PD-L1 positive CC samples was significantly higher than that in PD-L1 negative ones (p < 0.05). Moreover, the correlation between CD163 density and PD-L1 expression in TC was stronger. In order to determine the factors that predict the positive expression of PD-L1 in TC, we found that it was correlated with the reproductive history, abortion history, and the density of CD163-positive cells (Table 3). Subsequently, we carried out a multivariate analysis. The results showed that only the density of CD163-positive cells was correlated with the expression of PD-L1 in TC (odds ratio 1.52; p = 0.032; Table 3). This further indicated that the expression of PD-L1 in cervical squamous cell carcinoma was closely related to CD163+ M2 macrophage infiltration. The mechanism of TAMs activating PD-L1 expression in TME of cervical cancer needs to be further researched. Kasikara et al37 found that TAM receptor could up-regulate the expression of PD-L1 in breast cancer cell lines, suggesting that M2-like macrophage might be involved in the activation of PD-L1 expression in tumor cells. At the same time, it was found that IFN-γ was involved in the regulation of macrophage differentiation and PD-L1 expression in tumor cells, which played an important role in M2 TAM infiltration and PD-L1 expression in tumor tissues.38 PD-L1 was specifically expressed in tumor cells, and all kinds of cytokines could stimulate the up-regulation of PD-L1, in which IFN-γ is the most important stimulator to induce the up-regulation of PD-L1 expression.39,40 We are now working to evaluate the mechanism in our laboratory by which TAMs upregulated the expression of PD-L1 in cervical cancer cells. Given that more and more cancer patients can receive anti-PD-1/PD-L1 treatment, the effect of PD-1 blocking on macrophages in human cancer should not be ignored, as they may illuminate new disease indications or treatment combinations.
Conclusion
The expression of PD-L1 in TC was significantly different in the childbearing history and abortion history of the CC patients. The intratumoral density of CD163-positive cells was significantly different in the FIGO stage and lymph node metastasis. For the associations between them, the increase of PD-L1 expression in TC was significantly correlated with the increase of CD163 density. The increase of these two indicators was significantly correlated with short RFS in patients with cervical squamous cell carcinoma. To sum up, this study showed that CD163+ M2-like macrophage infiltration was highly associated with PD-L1 expression in TC, indicating that macrophage infiltration might serve as a potential therapeutic target.
Acknowledgment
We thank Jie Lv from Xinjiang Medical University for her assistance in checking the experimental results.
Funding Statement
This study was funded by the special scientific research project of young medical scientific and technological talents of Xinjiang Uygur Autonomous region Health and Family Planning Commission (WJWY-202154), the National Natural Science Foundation of China (81960467), Natural Science Foundation of Xinjiang Uygur Autonomous region (2019D01C260).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Naga CP, Gurram L, Chopra S, et al. The management of locally advanced cervical cancer. Curr Opin Oncol. 2018;30(5):323–329. doi: 10.1097/CCO.0000000000000471 [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3.Niyazi M, Sui S, Zhu K, et al. Correlation between methylation of human papillomavirus-16 L1 gene and cervical carcinoma in Uyghur women. Gynecol Obstet Invest. 2017;82(1):22–29. doi: 10.1159/000444585 [DOI] [PubMed] [Google Scholar]
- 4.Orbegoso C, Murali K, Banerjee S. The current status of immunotherapy for cervical cancer. Reports Pract Oncol Radiother. 2018;23(6):580–588. doi: 10.1016/j.rpor.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen JD, Du Pasquier L, Lefranc MP, et al. The B7 family of immunoregulatory receptors: a comparative and evolutionary perspective. Mol Immunol. 2009;46(3):457–472. doi: 10.1016/j.molimm.2008.10.007 [DOI] [PubMed] [Google Scholar]
- 6.Wang PF, Chen Y, Song SY, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. 2017;8(OCT):730. doi: 10.3389/fphar.2017.00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172 [DOI] [PubMed] [Google Scholar]
- 9.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung HC, Schellens JHM, Delord J-P, et al. Pembrolizumab treatment of advanced cervical cancer: updated results from the Phase 2 KEYNOTE-158 study. J Clin Oncol. 2018;36(suppl;abstr 5522). [Google Scholar]
- 13.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37–127ra37. doi: 10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultheis AM, Scheel AH, Ozretic L, et al. PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer. 2015;51(13):421–426. doi: 10.1016/j.ejca.2014.12.006 [DOI] [PubMed] [Google Scholar]
- 16.Bhatla N, Berek J, Cuello M, et al. New revised FIGO staging of cervical cancer (2018). Abstract S020.2. Presented at the FIGO XXII World Congress of Gynecology and Obstetrics. Rio de Janeiro, Brazil, October 14-19, 2018. Int J Gynecol Obstet. 2018;143(Suppl.3). doi: 10.1002/ijgo.12584 [DOI] [Google Scholar]
- 17.Mezache L, Paniccia B, Nyinawabera A, et al. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod Pathol. 2015;28(12):1594–1602. doi: 10.1038/modpathol.2015.108 [DOI] [PubMed] [Google Scholar]
- 18.Yang W, Lu YP, Yang YZ, et al. Expressions of programmed death (PD)-1 and PD-1 ligand (PD-L1) in cervical intraepithelial neoplasia and cervical squamous cell carcinomas are of prognostic value and associated with human papillomavirus status. J Obstet Gynaecol Res. 2017;43(10):1602–1612. doi: 10.1111/jog.13411 [DOI] [PubMed] [Google Scholar]
- 19.Li G, Lu C, Gao J, et al. Association between PD-1/PD-l1 and T regulate cells in early recurrent miscarriage. Int J Clin Exp Pathol. 2015;8(6):6512–6518. [PMC free article] [PubMed] [Google Scholar]
- 20.Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9(1):210–216. doi: 10.1186/1479-5876-9-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franklin RA, Liao W, Sarkar A, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344(6186):921–925. doi: 10.1126/science.1252510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang QW, Liu L, Gong CY, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7(12):e50946. doi: 10.1371/journal.pone.0050946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pei BX, Sun BS, Zhang ZF, et al. Interstitial tumor-associated macrophages combined with tumor-derived colony-stimulating factor-1 and interleukin-6, a novel prognostic biomarker in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2014;148(4):1208–1216. e2. doi: 10.1016/j.jtcvs.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 25.Carus A, Ladekarl M, Hager H, et al. Tumor-associated neutrophils and macrophages in non-small cell lung cancer: no immediate impact on patient outcome. Lung Cancer. 2013;81(1):130–137. doi: 10.1016/j.lungcan.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 26.Ma J, Liu L, Che G, et al. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10(1):112. doi: 10.1186/1471-2407-10-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kourea H, Kotoula V. Towards tumor immunodiagnostics. Ann Transl Med. 2016;4(14):263. doi: 10.21037/atm.2016.07.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Saleh W, Delvenne P, Arrese JE, et al. Inverse modulation of intraepithelial Langerhans’ cells and stromal macrophage/dendrocyte populations in human papillomavirus-associated squamous intraepithelial lesions of the cervix. Virchows Arch. 1995;427(1):41–48. doi: 10.1007/BF00203736 [DOI] [PubMed] [Google Scholar]
- 29.Davidson B, Goldberg I, Kopolovic J. Inflammatory response in cervical intraepithelial neoplasia and squamous cell carcinoma of the uterine cervix. Pathol Res Pract. 1997;193(7):491–495. doi: 10.1016/S0344-0338(97)80102-1 [DOI] [PubMed] [Google Scholar]
- 30.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27(4):462–472. doi: 10.1016/j.ccell.2015.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nedergaard BS, Ladekarl M, Thomsen HF, et al. Low density of CD3þ, CD4þ and CD8þ cells is associated with increased risk of relapse in squamous cell cervical cancer. Br J Cancer. 2007;97(8):1135–1138. doi: 10.1038/sj.bjc.6604001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33(3):119–126. doi: 10.1016/j.it.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Li G, Meng H, et al. Upregulation of B7-H1 expression is associated with macrophage infiltration in hepatocellular carcinomas. Cancer Immunol Immunother. 2012;61(1):101–108. doi: 10.1007/s00262-011-1094-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuang DM, Zhao Q, Peng C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206(6):1327–1337. doi: 10.1084/jem.20082173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartley GP, Chow L, Ammons DT, et al. Programmed Cell Death Ligand 1 (PD-L1) signaling regulates macrophage proliferation and activation. Cancer Immunol Res. 2018;6(10):1260–1273. doi: 10.1158/2326-6066.CIR-17-0537 [DOI] [PubMed] [Google Scholar]
- 36.Shima T, Shimoda M, Shigenobu T, et al. Infiltration of tumor-associated macrophages is involved in tumor programmed death-ligand 1 expression in early lung adenocarcinoma. Cancer Sci. 2020;111(2):727–738. doi: 10.1111/cas.14272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasikara C, Kumar S, Kimani S, et al. Phosphatidylserine sensing by TAM receptors regulates AKT-dependent chemoresistance and PD-L1 expression. Molecular Cancer Research. 2017;15(6):753–764. doi: 10.1158/1541-7786.MCR-16-0350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abiko K, Matsumura N, Hamanishi J, et al. IFN-c from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112(9):1501–1509. doi: 10.1038/bjc.2015.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4–328rv4. doi: 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3 potential mechanisms of action. Nat Rev Immunol. 2015;15(1):45–56. doi: 10.1038/nri3790 [DOI] [PubMed] [Google Scholar]