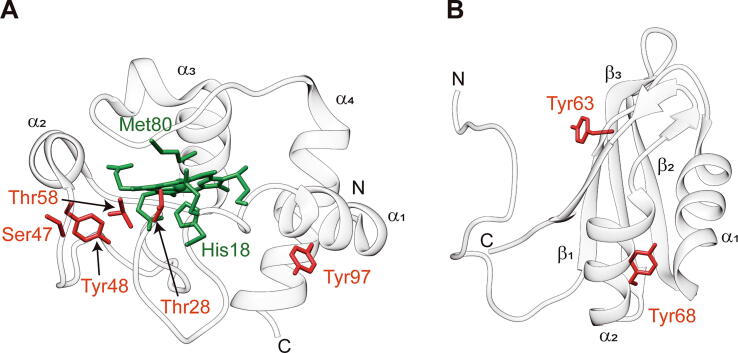
Fig. 1.
Phosphorylation sites of human cytochrome c and HuR. Richardson representations of human Cc (A; PDB ID: 2N9I; [142], [113]) and HuR1-101 (B; PDB ID: 5SZW; [118], [114]). Heme group and axial ligands (His18 and Met80) of Cc are colored in green. Phosphorylation sites described in the literature for mammalian Cc and HuR are highlighted in red. Thr at position 58 is the most conserved amino acid from bacteria to mammals, but it is not present in human Cc. N-terminal and C-terminal of the polypeptide chains are marked with N and C, respectively. Secondary structure elements (α-helix and β -sheet) are depicted. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).