Fig. 1.
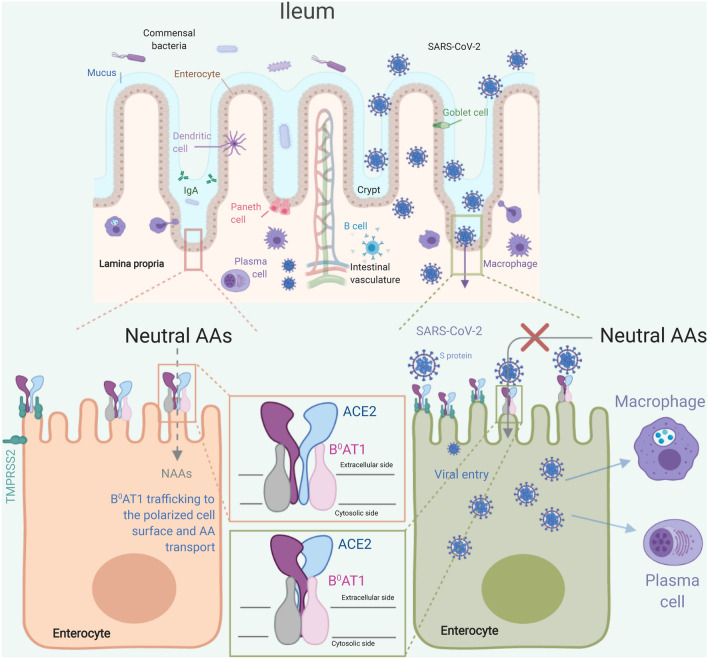
SARS-CoV-2 binding to ACE2 in the small intestine, principally ileum and jejunum, may cause functional changes of enterocytes and start the innate immune response. If host defense mechanisms are defective, massive viral replication may occur, leading to hyperinflammation and severe systemic complications. ACE2 also functions as the chaperone for surface expression of the amino acid transporter B0AT1, which mediates the uptake of neutral amino acids into ileal enterocytes. Mutations in B0AT1 cause Hartnup disorder, an inherited defect of amino acid transport, whose symptoms are reminiscent of those of COVID-19. Cryo–electron microscopy structure of the full-length human ACE2-B0AT1 complex has been recently described [23]. The ACE2 forms homodimers which are sandwiched by B0AT1, resulting in ACE2-B0AT1 complex. Structural analysis suggests that two SARS-CoV-2 spike (S) protein trimers simultaneously bind to an ACE2 homodimer. While the ACE2-B0AT1 complex exists in the open or closed state, the SARS-CoV-2-ACE2-B0AT1 ternary complex only displays the closed conformation. AA amino acids.
This figure was created by an author (E.N.) using the website https://app.biorender.com