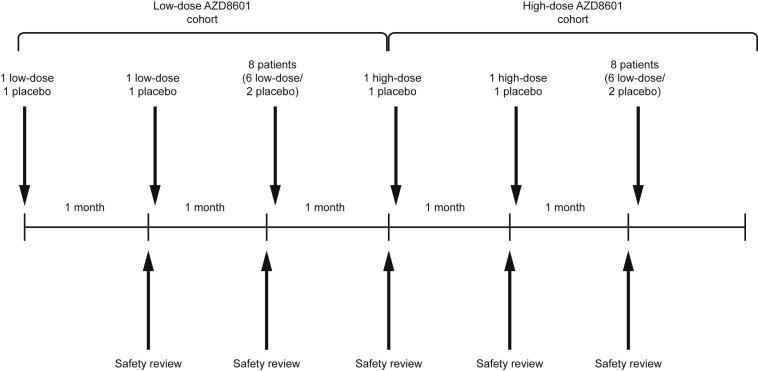
Figure 2.
Sentinel and Sequential Ascending-Dose Cohorts
Sequential low-dose and high-dose cohorts each include 12 participants, with 8 randomized to AZD8601 and 4 randomized to placebo. Each dose cohort includes 3 sequential sentinel sub-cohorts: the first and second sub-cohort each include 2 participants, randomized to AZD8601 (n = 1) or placebo (n = 1), and the third sentinel cohort includes 8 participants, randomized to AZD8601 (n = 6) or placebo (n = 2). Safety data at 1 month is reviewed before initiation of the next cohort or sub-cohort.