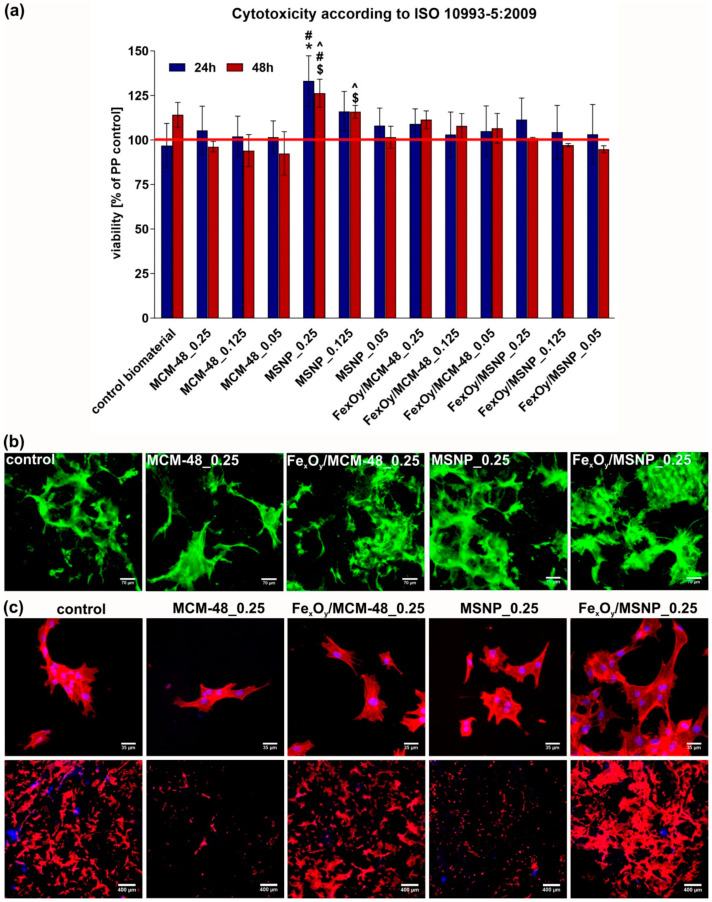
Figure 3.
Biocompatibility screening tests on the NPs-loaded biomaterials: (a) MTT cytotoxicity test with the use of extracts of the biomaterials (PP control—cells exposed to the extract of polypropylene, revealing 100% viability; control biomaterial—extract of the biomaterial without any NPs; 0.25, 0.125, 0.05—concentration (wt.%) of NPs within the structure of the biomaterial; * statistically significant differences compared to the control biomaterial, # compared to the biomaterial containing FexOy/MCM-48 at corresponding concentration; $ compared to the biomaterial containing MCM at a corresponding concentration, and ^ compared to the biomaterial containing FexOy/MSNPs at a corresponding concentration, according to One-way Anova followed by Tukey’s test, p < 0.05); (b) CLSM images of 3-day culture of preosteoblasts (MC3T3-E1 cells at high concentration were seeded) on the surface of the biomaterials upon fluorescent live/dead staining (control—biomaterial without any NPs; 0.25—concentration (wt.%) of NPs within the structure of the biomaterial; green fluorescence—viable cells, red fluorescence—nuclei of dead cells, magn. 200×); (c) CLSM images of 3-day culture of preosteoblasts (MC3T3-E1 cells at low concentration were seeded) on the surface of the biomaterials upon fluorescent staining of cytoskeleton and nuclei (red fluorescence—cytoskeleton, blue fluorescence—nuclei, upper images—magn. 400×, lower images—magn. 40×).