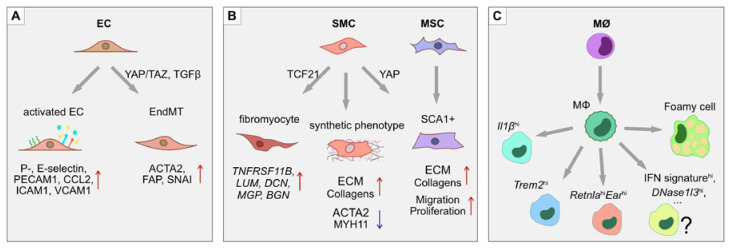
Figure 2.
Phenotypic switches during atherosclerosis. In atherosclerotic lesions, several processes of phenotypic modulation and trans-differentiation take place. (A) ECs upregulate adhesion molecules such as ICAM1, VCAM1, E- and P-selectins, as well as secreting pro-inflammatory cytokines CCL2 and IL1β, which help attract leukocytes. In atherosclerotic plaques, ECs also undergo endothelial-to-mesenchymal transition (EndMT) through the activation of YAP/TAZ- and TGFβ-driven pathways. The EndMT transitions are characterized by the loss of endothelial identity markers such as PECAM1, together with the upregulation of mesenchymal markers α-smooth muscle actin (ACTA2), fibroblast activation protein (FAP) and the SNAI transcription factors. (B) SMCs undergo a phenotypic switch from a contractile to a synthetic state by increasing production of ECM proteins and downregulating MYH11. Moreover, a subset of SMCs in the atherosclerotic lesion express the stem cell marker SCA1, suggesting either mesenchymal stem cell differentiation into SMCs, or de-differentiation of SMCs towards an MSC-like state. (C) Monocytes, upon transmigration into the intima of the lesion, differentiate into macrophages that display at least 3 unique subsets: (i) resident-like anti-inflammatory cells, (ii) pro-inflammatory Il1βhi cells, and (iii) Trem2hi cells. Macrophages that take up low-density lipoproteins (LDLs) upregulate lipid metabolism related genes and take on a “foamy macrophage” phenotype.